
(a)
Interpretation:
The quantity which remains constant in Boyle’s law, Charles’s law and
Concept Introduction:
Boyle’s law, Charles’s law and Avogadro’s law describe the relationship between two variables keeping one variable constant.
(a)
Answer to Problem 5RQ
In Boyle’s law, temperature remains constant.
In Charles’s law, Pressure remains constant.
In
Explanation of Solution
Boyle’s law: The pressure of a gas is inversely proportional to the volume of the gas at constant temperature.
The relationship between pressure and volume of a gas is expressed as-
Charles’s law: The volume of a given amount of gas is proportional to its temperature (in K) of the gas at constant pressure.
The relationship between volume of a gas and temperature is expressed as-
The relationship between the volume of a gas and the number of moles present in the gas is expressed as-
So,
| Boyle’s law | Charles’s law | Avogadro’s law | |
| Constants | Temperature | Pressure | Pressure and temperature |
(b)
Interpretation:
The quantities which are variables in Boyle’s law, Charles’s law and
Concept Introduction:
Boyle’s law, Charles’s law and Avogadro’s law describe the relationship between two variables keeping one variable constant.
(b)
Answer to Problem 5RQ
In Boyle’s law, Pressure and volume are variables.
In Charles’s law, Volume and temperature are variables.
In
| Boyle’s law | Charles’s law | Avogadro’s law | |
| Variables | Pressure and volume | Volume and temperature | Volume and number of moles |
Explanation of Solution
Boyle’s law: The pressure of a gas is inversely proportional to the volume of the gas at constant temperature.
The relationship between pressure and volume of a gas is expressed as-
Charles’s law: The volume of a given amount of gas is proportional to its temperature (in K) of the gas at constant pressure.
The relationship between volume of a gas and temperature is expressed as-
The relationship between the volume of a gas and the number of moles present in the gas is expressed as-
| Boyle’s law | Charles’s law | Avogadro’s law | |
| Variables | Pressure and volume | Volume and temperature | Volume and number of moles |
(c)
Interpretation:
The graph of Boyle’s law, Charles’s law and
Concept Introduction:
Boyle’s law, Charles’s law and Avogadro’s law describe the relationship between two variables keeping one variable constant.
(c)
Answer to Problem 5RQ
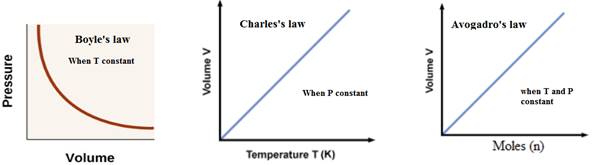
Explanation of Solution
Boyle’s law: The pressure of a gas is inversely proportional to the volume of the gas at constant temperature.
The relationship between pressure and volume of a gas is expressed as-
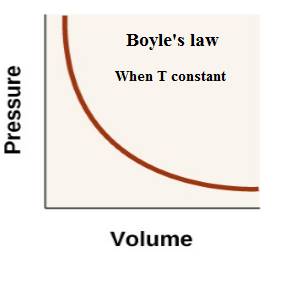
Charles’s law: The volume of a given amount of gas is proportional to its temperature (in K) of the gas at constant pressure.
The relationship between volume of a gas and temperature is expressed as-
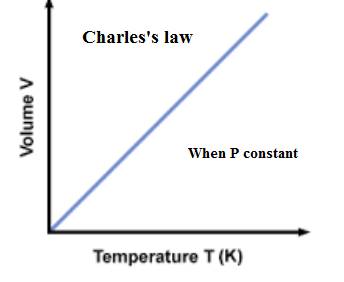
The relationship between the volume of a gas and the number of moles present in the gas is expressed as-
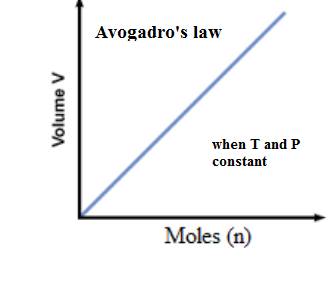
(d)
Interpretation:
Boyle’s law, Charles’s law and
Concept Introduction:
Boyle’s law, Charles’s law and Avogadro’s law describe the relationship between two variables keeping one variable constant.
(d)
Answer to Problem 5RQ
Volume of a gas is directly proportional to its temperature and the amount of gas.
Charles’s law showed that the volume of a gas is directly proportional to its temperature (in K) at constant pressure.
Explanation of Solution
Boyle’s law:
Charles’s law:
So, volume of a gas is directly proportional to its temperature and the amount of gas. Charles’s law showed that the volume of a gas is directly proportional to its temperature (in K) at constant pressure and Avogadro’s law showed that the volume of a given amount of gas is directly related to the number of moles at constant temperature and pressure.
Chapter 13 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





