
Concept explainers
A spring-loaded piston–cylinder device contains a mixture of gases whose pressure fractions are 25 percent Ne, 50 percent O2, and 25 percent N2. The piston diameter and spring are selected for this device such that the volume is 0.1 m3 when the pressure is 200 kPa and 1.0 m3 when the pressure is 1000 kPa. Initially, the gas is added to this device until the pressure is 200 kPa and the temperature is 10°C. The device is now heated until the pressure is 500 kPa. Calculate the total work and heat transfer for this process.
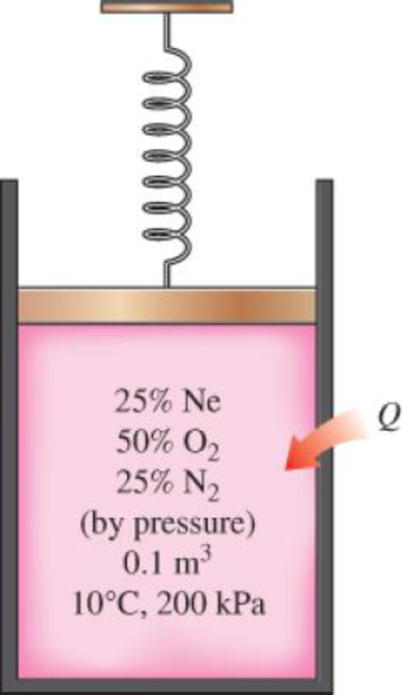
The total work done and heat transfer for the process.
Answer to Problem 94RP
The total work done for the process is
The heat transfer for the process is
Explanation of Solution
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the mass of
Write the expression to calculate the mass of
Write the expression to calculate the mass of
Write the expression to calculate the total mass of each component
Write the expression to calculate the mass fraction of
Write the expression to calculate the mass fraction of
Write the expression to calculate the mass fraction of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the total number of moles
Write the expression to calculate the apparent molecular weight of the mixture
Write the expression to calculate the constant volume specific heat of the mixture
Here, mole fraction of
Write the expression to calculate the apparent gas constant of the mixture
Here, universal gas constant is
Write the expression for the mass contained in the system
Write the expression to calculate the final temperature
Write the expression to calculate the work done during the process.
Conclusion:
From Table A-1, “Molar mass, gas constant, and critical point properties”, obtain the values of molar masses for
From Table A-2a, “Ideal-gas specific heats of various common gases”, obtain the following properties for
For
For
For
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
The pressure changes linearly with volume as shown is Figure (1).
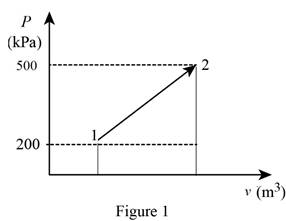
Using the data form Prob. 13–94 obtain the value of final volume by linear interpolation.
Write the straight line equation for two points.
Here, coordinates of the point 1 is
Substitute
The final volume
Substitute
Substitute
Thus, the total work done for the process is
Write a energy balance on the system.
Here, input energy transfer and output energy transfer is
The rate of change in energy of a system
For given system the energy balance Equation (XXII) is expressed as follows:
The rate of change in energy of a system
Substitute
Thus, the heat transfer for the process is
Want to see more full solutions like this?
Chapter 13 Solutions
Thermodynamics: An Engineering Approach
- On a certain day, the temperature and relative humidity of air over a large swimming pool are measured to be 25°C and 60 percent, respectively. Determine the water temperature of the pool when phase equilibrium conditions are established between the water in the pool and the vapor in the air.arrow_forwardDetermine the internal energy change for carbon monoxide, in kJ/kg, as it is heated from 312° K to 1456° K, using the ideal gas properties tablearrow_forwardWater is boiled at sea level in a coffeemaker equipped with an immersion-type electric heating element. The coffeemaker contains 1 L of water when full. Once boiling starts, it is observed that half of the water in the coffeemaker evaporates in 13 min. Determine the power rating of the electric heating element immersed in water. Also, determine how long it will take for this heater to raise the temperature of 1 L of cold water from 18°C to the boiling temperature.arrow_forward
- A 300 m3 rigid tank is filled with saturated liquid-vapor mixture of water at 200 kPa. If 25 percent of the mass is liquid and 75 percent of the mass is vapor, determine the total mass in the tank.arrow_forwardIn order to cool 1 ton of water at 20°C in an insulated tank, a person pours 140 kg of ice at –5°C into the water. Determine the final equilibrium temperature in the tank.arrow_forwardA 1-kg steam-water mixture at 1.0 MPa is contained in an inflexible tank. Heat is added until the pressure rises to 3.5 MPa and the temperature to 400°C. Determine the heat added in kJ.arrow_forward
- A cylinder contains a mixture of air and wet steam at a pressure of 130kN/m2 and a temperature of 760 C. The dryness fraction of the steam is 0.92. The air – steam mixture is then compressed to one-fifth of its original volume the final temperature being 1250 C. Determine: a) The final pressure in the cylinder b) The final dryness fraction of the steam. Note: I need both right solutions.arrow_forwarda. 9.23 kg of water vapor is contained at 150 kPa and 90 percent quality in a suitable enclosure. Calculate the heat in kJ which must be added to produce a saturated vapor.b. 9.96 kg of water vapor are contained at 150 kPa and 90 percent quality in a suitable enclosure. What will the pressure be in MPa at the end of the heating process?Please include the h-s and T-s diagram in the solution, and do not round off intermediate answers only the final answer in three decimal places.arrow_forwardThe piston in a car engine compresses a mixture of gasoline vapour and air from 700ml to 100ml during its compression stroke. Before the compression stroke, the mixture had a pressure of 75 kPa and a temperature of 120celcius. What is the pressure of the cylinder after the compression stroke if the temperature of the gas rises to 200celcius? Express your answer in MPa with two significant digits.arrow_forward
- Ten pounds of water at 35oF, and 6.00 lb of steam at 250oF and 20 psia are mixed together in a container of fixed volume. What is the final temperature of the mixture? How much steam condense? Assume that the volume of the vessel is constant with a value equal to the volume of the steam and that the vessel is insulated.arrow_forwardA gas mixture of oxygen and nitrogen has an oxygen mass fraction of 0.1. The mixture is heated at a constant pressure of 333 kPa in a closed system from a temperature of 62 C to a final temperature of 213 C. Calculate the work done in kJ/kg.arrow_forwardTen kilograms of steam at 1.1 MPa has an internal energy of 31,228 kJ. Determine a) its temperature, b) Total enthalpy.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





