
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.13QAP
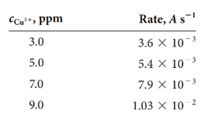
Copper(II) forms a 1:1 complex with the organic complexing agent R in acidic medium. The formation ofthe complex can be monitored by spectrophotometry at 480 nm. Use the following data collected under pseudo-first-order conditions to construct a calibration curve of rate versus concentration of R. Find the concentration of copper(II) in an unknown whose rate under the same conditions was 6.2 × 10- 3A s-1.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The following data were obtained on the initial rates of reaction of a d-metal complex in aqueous solution.
For the experiments a. [Y] = 2.7 mmol/dm3,
and for experiments b. [Y] = 6.1 mmol/dm3
Conc. complex/(mmol dm-3)
8.01
9.22
12.11
Exp. a. v/ (mol dm-3 s-1)
125
144
190
Exp. b. v/ (mol dm-3 s-1)
640
730
960
(a) What is the order of reaction with respect to the complex AND the reactant Y. Choose one.
(b) use the data above to calculate the rate constant.
k = ________ (mol/L/s, s-1, L/mol/s, or L2/mol2/s). 3 s.f. normal format.
The following data were obtained on the initial rates of reaction of a d-metal complex in aqueous solution.
For the experiments a. [Y] = 2.7 mmol/dm3,
and for experiments b. [Y] = 6.1 mmol/dm3
Conc. complex/(mmol dm-3)
8.01
9.22
12.11
Exp. a. v/ (mol dm-3 s-1)
125
144
190
Exp. b. v/ (mol dm-3 s-1)
640
730
960
(a) What is the order of reaction with respect to the complex AND the reactant Y.
(b) use the data above to calculate the rate constant.
k = ________ (mol/L/s, s-1, L/mol/s, or L2/mol2/s). 3 s.f. normal format.
The following data were obtained on the initial rates of reaction of a d-metal complex in aqueous solution.
For the experiments a. [Y] = 2.7 mmol/dm3,
and for experiments b. [Y] = 6.1 mmol/dm3
Conc. complex/(mmol dm-3)
8.01
9.22
12.11
Exp. a. v/ (mol dm-3 s-1)
125
144
190
Exp. b. v/ (mol dm-3 s-1)
640
730
960
(a) What is the order of reaction with respect to the complex AND the reactant Y. Choose one.
Chapter 14 Solutions
Principles of Instrumental Analysis
Ch. 14 - Prob. 14.1QAPCh. 14 - A 0.4740-g pesticide sample was decomposed by wet...Ch. 14 - Sketch a photometric titration curve for the...Ch. 14 - Prob. 14.4QAPCh. 14 - Prob. 14.5QAPCh. 14 - The accompanying data (1.00-cm cells) were...Ch. 14 - A 3.03-g petroleum specimen was decomposed by wet...Ch. 14 - Prob. 14.8QAPCh. 14 - Prob. 14.9QAPCh. 14 - The acid-base indicator HIn undergoes the...
Ch. 14 - Prob. 14.11QAPCh. 14 - Prob. 14.12QAPCh. 14 - Copper(II) forms a 1:1 complex with the organic...Ch. 14 - Aluminum forms a 1:1 complex with...Ch. 14 - Prob. 14.15QAPCh. 14 - Prob. 14.16QAPCh. 14 - Prob. 14.17QAPCh. 14 - Prob. 14.18QAPCh. 14 - Prob. 14.19QAPCh. 14 - Given the Information that...Ch. 14 - Prob. 14.21QAPCh. 14 - Mixing the chelating reagent B with Ni(II) forms...Ch. 14 - Prob. 14.23QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aluminum forms a 1:1 complex with 2-hydroxy-1-naphthaldehyde p -methoxybenzoylhydraxonal, which absorbs UV radiation at 285 nm. Under pseudo-first-order conditions, a plot of the initial rate of the reaction (absorbance units per second) versus the concentration of aluminum (in M) yields a straight linedescribed by the equation rate =1.92 c Al- 0.250 Find the concentration of aluminum in a solution that exhibits a rate of 0.53 absorbance units per second under the same experimental conditions.arrow_forwardThe following data were obtained on the initial rates of reaction of a d-metal complex in aqueous solution. For the experiments a. [Y] = 2.7 mmol/dm3, and for experiments b. [Y] = 6.1 mmol/dm3 Conc. complex/(mmol dm-3) 8.01 9.22 12.11 Exp. a. v/ (mol dm-3 s-1) 125 144 190 Exp. b. v/ (mol dm-3 s-1) 640 730 960 b) use the data above to calculate the rate constant. k = ________ (mol/L/s, s-1, L/mol/s, or L2/mol2/s). 3 s.f. normal format.arrow_forward. A student collects the following data for the absorbance of a complex measured in 1.00 cmspectrophotometric cells. The molar absorptivity (ε) of the complex under investigation is 2.25×103L/mol·cm. Calculate the concentration of the complex in solution at each time of the reaction.Time (min)MeasuredAbsorbanceComplex Concentration(M)0.000 1.50010.000 1.31320.000 1.12530.000 0.93840.000 0.750arrow_forward
- please help me answer my assignment. thanks the rate constants (k) for the leaching of galena in an aqeous medium containing ammonium acetate under oxygen pressure at the different temperature are listed below: log K -10.9 -11.1 -11.2 -11.5 -12.1 -12.6 1/t x10^3 2.30 2.35 2.38 2.45 2.61 2.76 where k and T are expressed in mole^2/cm/min and K respectively. Calculate the activation energy of the leaching processarrow_forwardExplain why the velocity constant (k) of the complex reaction[Co(NH3)5N3]2+ with [Cr(H2O)6]2+ is on the order of 3x105 while for [Co(NH3)6]3+ with [Cr(H2O)6]2+ is 9x10-5 .Indicate the steps of the proposed mechanism and the species involved.arrow_forwardThe molecu le 2,2'-bipyridine (1, bpy) forms a complex with the Ru2+ ion. Ruthenium(ll) tris-(2,2'-bipyridyl), Ru(bpy)3,2+(2), has a strong electronic absorpt ion at 450 nm . The quenching of the * Ru(bpy)3 2+ excited state by Fe(OH2)63+. in acidic solution was monitored by measuring excited state lifetimes at 600 nm. Determine the quenching rate constant for this reaction from the following data:arrow_forward
- What is the activation energy (Qv) in eV/atom for a vancay formation if 10 moles of a metal has 2.3x10 ^ 13 vacancies at 425 C?arrow_forwardWhat term most accurately describes the process below? a. hydrogen abstraction b. halogen abstraction c. initiation d. couplingarrow_forwardCalculate the concentration of vacancies in in fcc silver at 250 oC given that the activation energy for vacancy creation in silver is 79.5 kJ/mol. The units for your answer should be vacancies/cm3arrow_forward
- The viscosity of water at 25oC is 2.890´10-2 Kg/m/s. Calculate the value of activation energy (Ea) for molecular motion in the solution. (ho= 1.547´10-2 Kg/m/s; R=8.314 J/K/mol)arrow_forwardPalladium (II) and gold (III) can be analyzed simultaneously through reaction with methiomeprazine (C19H24N2S2). The absorption maximum for the Pd complex occurs at 480 nm, while that for the Au complex is at 635 nm. Molar absorptivity data at these wavelengths are Molar Absorptivity, ε 480 nm 635 nm Pd complex 3.55 × 103 5.64 × 102 Au complex 2.96 × 103 1.45 × 104 A 25.0-mL sample was treated with an excess of methiomeprazine and subsequently diluted to 50.0 mL. Calculate the molar concentrations of Pd(II), CPd, and Au(III), CAu, in the sample if the diluted solution had an absorbance of 0.533 at 480 nm and 0.590 at 635 nm when measured in a 1.00-cm cell. explain each steparrow_forward6. The reaction of [Co(NH3)4Cl2]+ with H2O is 103 times faster than the reaction of [Co(NH3)5Cl]2+. By considering how the charge on a complex affects bond strengths,discuss briefly whether this data is consistent with a Id or Ia mechanismarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License