
Concept explainers
(a)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
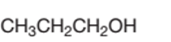
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be
Answer to Problem 14.29P
Propanol is a primary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here, the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a primary C atom which is bonded with one other C atom hence, it should be primary alcohol.
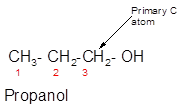
(b)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
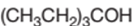
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
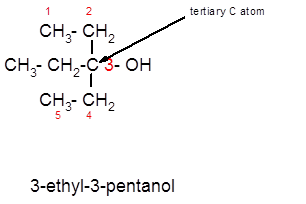
3-ethyl-3-pentanol is a tertiary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a tertiary C atom which is bonded with 3 other C atoms hence, it should be tertiary alcohol.
(c)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
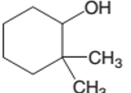
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
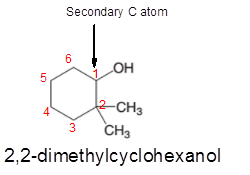
2,2-dimethylcyclohexanol is a secondary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a secondary C atom which is bonded with two other C atoms hence, it should be secondary alcohol.
(d)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
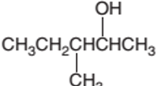
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
3-methyl-2-pentanol is a secondary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group bonds to a secondary C atom which is bonded with two other C atoms hence it should be secondary alcohol.
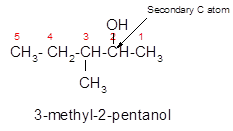
Want to see more full solutions like this?
Chapter 14 Solutions
General, Organic, & Biological Chemistry
- Give both the IUPAC name and the common name for each alcohol.(a) CH3CH2CH(OH)CH3arrow_forwardWhat products are formed when each alcohol is oxidized with K 2Cr 2O 7? In some cases, no reaction occurs.arrow_forwardWhich of the following statements concerning the physicalproperties of alcohols is incorrect a. Alcohol solubility in water decreases as the carbon chain length increases b. Alcohol solubility in water decreases as the number of -OH groups present increases c. Alcohol boiling points increases as C chain length increases. d. C1 to C4 straight-chain alcohols are liquids at room temperaturearrow_forward
- Draw the products formed when each alcohol is oxidized with K 2Cr 2O 7. In some cases, no reaction occurs.arrow_forwardClassify each statement as a property of ethers, alcohols, or both ethers and alcohols. 1. These compounds cannot form hydrogen bonds between themselves, but they can form hydrogen bonds with other compounds containing an O−HO−H , N−HN−H , or F−HF−H bond, such as water.2. These compounds are water soluble if they have fewer than four carbon atoms.3. When comparing ethers and alcohols of similar molecular weights, these compounds have the higher boiling point. alcohols both ethers and alcohols ethersarrow_forwardWhich of the following alcohols is least likely to be soluble in water? 3-pentanol 2-butanol 1,2-ethanediol 1,2,3-propanetriol Methanolarrow_forward
- Differentiate the three types of alcohols. Give an example of eacharrow_forwardWhat kind of solvent ingredients is usually used in the concentrations of 4-10 percent in skin care products and their function is to soften skin cells and to lessen wrinkles? A. Ethly acetate B. Alpha hydroxyl acids C. Phenols and phenol derivatives D. Aliphatic alcoholsarrow_forwardSeveral important alcohols are well known by common names. Give a common name for each of the following: a. CH3OH b. c. CH3CH2OH d. e.arrow_forward
- Determine the maximum number of hydrogen bonds that can form between an ethanol molecule and a. other ethanol molecules b. water molecules c. methanol molecules d. 1-propanol moleculesarrow_forwardArrange these compounds in order of increasing boiling point. (a) 1-butanol, butane, diethylether (b) hexane, 1-hexanol, dipropyletherarrow_forwardAssign an IUPAC name to each of the following compounds. a. CH3(CH2)4SH b. CH3(CH2)4OHarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





