
Study Guide And Full Solutions Manual For Fundamentals Of General, Organic, And Biological Chemistry
8th Edition
ISBN: 9780134261379
Author: McMurry, John E., BALLANTINE, David S., HOEGER, Carl A., Peterson, Virginia E., Susan
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.33AP
Give systematic names for the following compound
(a) 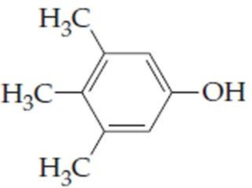
(b) 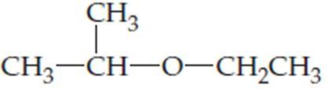
(c) 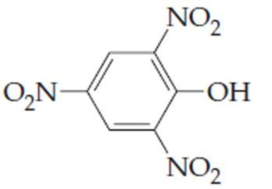
(Also known as picric acid)
(d) 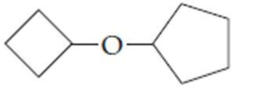
(e) 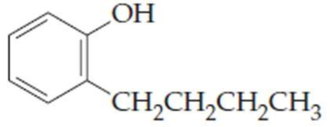
(f) CH3CH2CH2OCH2CH2CH3
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw all possible carboxylic acids with the formula C5H10O2.
What is the empirical formula for C3H6O3?
C3H6O3
C6H12O6
CH2O
None of these
Give IUPAC names for the five isomers with the formula C6H14.
Chapter 14 Solutions
Study Guide And Full Solutions Manual For Fundamentals Of General, Organic, And Biological Chemistry
Ch. 14.1 - Identify each of the following compounds as an...Ch. 14.1 - Prob. 14.2PCh. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Prob. 14.6PCh. 14.3 - For each of the following molecules, (i) redraw...Ch. 14.4 - Prob. 14.1MRPCh. 14.4 - Provide the mechanism for the dehydration of...Ch. 14.4 - Prob. 14.3MRP
Ch. 14.4 - Prob. 14.8PCh. 14.4 - What alcohols yield the following alkenes as the...Ch. 14.4 - Prob. 14.10KCPCh. 14.4 - What products would you expect from oxidation of...Ch. 14.4 - Prob. 14.12PCh. 14.4 - Prob. 14.13KCPCh. 14.5 - Prob. 14.14PCh. 14.5 - Prob. 14.15PCh. 14.7 - Prob. 14.1CIAPCh. 14.7 - Prob. 14.2CIAPCh. 14.7 - Prob. 14.3CIAPCh. 14.7 - Prob. 14.16PCh. 14.8 - What disulfides would you obtain from oxidation of...Ch. 14.9 - Prob. 14.18PCh. 14.10 - Prob. 14.19PCh. 14.10 - Prob. 14.20PCh. 14.10 - Prob. 14.4CIAPCh. 14.10 - Prob. 14.5CIAPCh. 14.10 - Prob. 14.6CIAPCh. 14.10 - Prob. 14.7CIAPCh. 14 - Prob. 14.21UKCCh. 14 - Prob. 14.22UKCCh. 14 - Prob. 14.23UKCCh. 14 - Prob. 14.24UKCCh. 14 - Prob. 14.25UKCCh. 14 - How do alcohols, ethers, and phenols differ...Ch. 14 - What is the structural difference between primary,...Ch. 14 - Prob. 14.28APCh. 14 - Prob. 14.29APCh. 14 - The Taxane nucleus is shown here; it is the basis...Ch. 14 - Vitamin E has the structure shown. Identify the...Ch. 14 - Give systematic names for the following alcohols:...Ch. 14 - Give systematic names for the following compound...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Prob. 14.36APCh. 14 - Locate the alcohol functional groups in the taxane...Ch. 14 - Prob. 14.38APCh. 14 - Prob. 14.39APCh. 14 - Prob. 14.40APCh. 14 - Prob. 14.41APCh. 14 - Prob. 14.42APCh. 14 - Prob. 14.43APCh. 14 - Assume that you have samples of the following two...Ch. 14 - Which of the following alcohols can undergo...Ch. 14 - The following alkenes can be prepared by...Ch. 14 - Prob. 14.47APCh. 14 - Prob. 14.48APCh. 14 - What alcohols would you oxidize to obtain the...Ch. 14 - Prob. 14.50APCh. 14 - What is the structural relationship between a...Ch. 14 - Prob. 14.52APCh. 14 - Prob. 14.53APCh. 14 - Prob. 14.54APCh. 14 - Prob. 14.55APCh. 14 - Prob. 14.56APCh. 14 - Prob. 14.57APCh. 14 - Identify the chiral center(s) in each of the...Ch. 14 - Are the following molecules chiral or achiral? If...Ch. 14 - Prob. 14.60CPCh. 14 - Prob. 14.61CPCh. 14 - 1-Propanol is freely soluble in water, 1-butanol...Ch. 14 - Prob. 14.63CPCh. 14 - Prob. 14.64CPCh. 14 - Prob. 14.65CPCh. 14 - Prob. 14.66CPCh. 14 - Prob. 14.67CPCh. 14 - Prob. 14.68CPCh. 14 - Prob. 14.69CPCh. 14 - Prob. 14.70CPCh. 14 - Prob. 14.71CPCh. 14 - Prob. 14.72CPCh. 14 - (a)Draw all possible cyclic C7H14O alcohol isomers...Ch. 14 - Prob. 14.74GPCh. 14 - Prob. 14.75GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- 1-34. Indicate whether each of the following statements about enantiomers is true or false. (a to d)arrow_forwardName the following molecular: CH3-CH2-CH2-CH2-CH2-CH-OHarrow_forwardDraw the full structure of triglyceride 1-docosahexanoyl-2-arachidonyl-3-elaidyl-glycerol with proper stereochemistry, given the following information: docosahexanoic acid: 22:6n-3, arachidonic acid: 20:4n-6, elaidic acid: trans-18:1n-9,arrow_forward
- Draw structures corresponding to the following names:(a) 3-Methylhexan-1-ol (b) 1-Methyl-3-propylcyclopentanol(c) 2,2-Dimethylhexan-3-ol (d) Heptan-3-ol(e) 2,3-Diethylcyclohexanolarrow_forwardWhat is the molar mass of diazepam (Valium), C16H13ClN2O?arrow_forwardGlutathione, a powerful antioxidant that destroys harmful oxidizing agents in cells, is composed of glutamic acid, cysteine, and glycine, and has the following structure. a.) What product is formed when glutathione reacts with an oxidizing agent?b.) What is unusual about the peptide bond between glutamic acid and cysteine?arrow_forward
- Consider the intermolecular forces present in a pure sample of each of the following compounds: CH₃CH₂OH and CH₃COCH₃. Identify the intermolecular forces that these compounds have in common.arrow_forwardGlucose-1-phosphate has a ΔG°′ value of −20.9 kJ/mol, whereas that for glucose-6-phosphate is −12.5 kJ/mol. After reviewing the molecular structures of these compounds, explain why there is such a difference in these values.arrow_forwardDraw the complete structural formula of arachidonic acid (Table 23.1) in a way that shows the cis stereochemistry of its four double bonds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license