
Study Guide And Full Solutions Manual For Fundamentals Of General, Organic, And Biological Chemistry
8th Edition
ISBN: 9780134261379
Author: McMurry, John E., BALLANTINE, David S., HOEGER, Carl A., Peterson, Virginia E., Susan
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.3, Problem 14.7P
For each of the following molecules, (i) redraw using line structure format, (ii) identify its hydrophobic and hydrophilic parts, and (iii) predict its solubility in water.
(a) CH3(CH2)10CH2OH
(b) 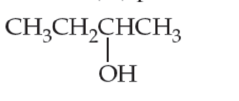
(c) 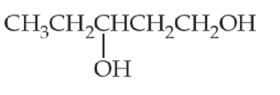
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What structural and functional advantages do proteins gain by associating to form quaternary structures?
Which structure is saturated and unsaturated?
What are the molarity and the normality of a solution made by dissolving 25 g of citric acid (triprotic, C6H5O7H3) in enough water to make 800 mL of solution?
Chapter 14 Solutions
Study Guide And Full Solutions Manual For Fundamentals Of General, Organic, And Biological Chemistry
Ch. 14.1 - Identify each of the following compounds as an...Ch. 14.1 - Prob. 14.2PCh. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Prob. 14.6PCh. 14.3 - For each of the following molecules, (i) redraw...Ch. 14.4 - Prob. 14.1MRPCh. 14.4 - Provide the mechanism for the dehydration of...Ch. 14.4 - Prob. 14.3MRP
Ch. 14.4 - Prob. 14.8PCh. 14.4 - What alcohols yield the following alkenes as the...Ch. 14.4 - Prob. 14.10KCPCh. 14.4 - What products would you expect from oxidation of...Ch. 14.4 - Prob. 14.12PCh. 14.4 - Prob. 14.13KCPCh. 14.5 - Prob. 14.14PCh. 14.5 - Prob. 14.15PCh. 14.7 - Prob. 14.1CIAPCh. 14.7 - Prob. 14.2CIAPCh. 14.7 - Prob. 14.3CIAPCh. 14.7 - Prob. 14.16PCh. 14.8 - What disulfides would you obtain from oxidation of...Ch. 14.9 - Prob. 14.18PCh. 14.10 - Prob. 14.19PCh. 14.10 - Prob. 14.20PCh. 14.10 - Prob. 14.4CIAPCh. 14.10 - Prob. 14.5CIAPCh. 14.10 - Prob. 14.6CIAPCh. 14.10 - Prob. 14.7CIAPCh. 14 - Prob. 14.21UKCCh. 14 - Prob. 14.22UKCCh. 14 - Prob. 14.23UKCCh. 14 - Prob. 14.24UKCCh. 14 - Prob. 14.25UKCCh. 14 - How do alcohols, ethers, and phenols differ...Ch. 14 - What is the structural difference between primary,...Ch. 14 - Prob. 14.28APCh. 14 - Prob. 14.29APCh. 14 - The Taxane nucleus is shown here; it is the basis...Ch. 14 - Vitamin E has the structure shown. Identify the...Ch. 14 - Give systematic names for the following alcohols:...Ch. 14 - Give systematic names for the following compound...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Draw structures corresponding to the following...Ch. 14 - Prob. 14.36APCh. 14 - Locate the alcohol functional groups in the taxane...Ch. 14 - Prob. 14.38APCh. 14 - Prob. 14.39APCh. 14 - Prob. 14.40APCh. 14 - Prob. 14.41APCh. 14 - Prob. 14.42APCh. 14 - Prob. 14.43APCh. 14 - Assume that you have samples of the following two...Ch. 14 - Which of the following alcohols can undergo...Ch. 14 - The following alkenes can be prepared by...Ch. 14 - Prob. 14.47APCh. 14 - Prob. 14.48APCh. 14 - What alcohols would you oxidize to obtain the...Ch. 14 - Prob. 14.50APCh. 14 - What is the structural relationship between a...Ch. 14 - Prob. 14.52APCh. 14 - Prob. 14.53APCh. 14 - Prob. 14.54APCh. 14 - Prob. 14.55APCh. 14 - Prob. 14.56APCh. 14 - Prob. 14.57APCh. 14 - Identify the chiral center(s) in each of the...Ch. 14 - Are the following molecules chiral or achiral? If...Ch. 14 - Prob. 14.60CPCh. 14 - Prob. 14.61CPCh. 14 - 1-Propanol is freely soluble in water, 1-butanol...Ch. 14 - Prob. 14.63CPCh. 14 - Prob. 14.64CPCh. 14 - Prob. 14.65CPCh. 14 - Prob. 14.66CPCh. 14 - Prob. 14.67CPCh. 14 - Prob. 14.68CPCh. 14 - Prob. 14.69CPCh. 14 - Prob. 14.70CPCh. 14 - Prob. 14.71CPCh. 14 - Prob. 14.72CPCh. 14 - (a)Draw all possible cyclic C7H14O alcohol isomers...Ch. 14 - Prob. 14.74GPCh. 14 - Prob. 14.75GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the hydrolyzed component of the structure below?arrow_forwardBeyond the differences in molecular mass, what is different about determining the molarity of a solution containing a salt (e.g. NaCl, MgCl2) versus a covalently linked molecule (e.g. glucose)?arrow_forwardDraw the condensed structural formula for the fatty acid whose numerical shorthand designation is 18:2 (△ 9,12)arrow_forward
- How many grams of glucose (C6H2O6 molecular mass =180daltons) would be present in one liter of a 1M (molar) solution of glucose?arrow_forwardWhat structural features does a sphingolipid have in common with proteins? Are there functional similarities?arrow_forwardFor each of the following chemicals, name the general class they belong to, discuss their solubility in water and explain why they are/are not soluble in water (note the numbers are all subscripts): a) CH3(CH2)26COOH b) KCl c) CH3OHarrow_forward
- Draw condensed structural formulas for the two carboxylic acids with the molecular formula C4H8O2arrow_forwardWhat intermolecular forces hold protein subunits in a quaternary structure?arrow_forwardWhat are the functional groups in biological macromolecules that contribute to ionic bonding interactions between macromolecules?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Metabolic Pathways; Author: Wisc-Online;https://www.youtube.com/watch?v=m61bQYio9ys;License: Standard Youtube License