
Concept explainers
a.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
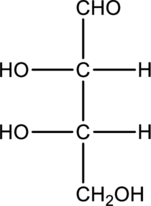
Concept Introduction:
A carbon atom that is bonded to four different groups is known as a chiral carbon atom. This can rotate the plane polarized light. D- and L- isomers of monosaccharide can be identified by looking into the chiral center that is farther from the carbonyl group. In a Fischer projection, if the
Enantiomers are two stereoisomers of a compound that rotate the plane polarized light exactly opposite. The configuration present in the enantiomers will be exactly opposite to each other.
Fischer projection formula simply uses cross for representing tetrahedral carbon atom. The carbon atom that is present in the intersection point in cross. The horizontal bonds means they are coming forward and they are present on wedge bond. The vertical bonds means they are pointing away and they are present on dashed lines.
b.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
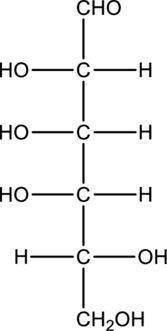
Concept Introduction:
Refer part “a.”.
c.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
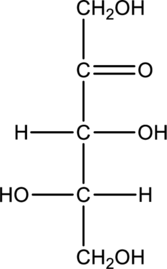
Concept Introduction:
Refer part “a.”.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





