
Principles of General Organic & Biological Chemistry
2nd Edition
ISBN: 9780077633721
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14, Problem 14.54AP
Interpretation Introduction
Interpretation:
Product that will be formed when the given aldopentose is treated with Benedict’s reagent has to be drawn.
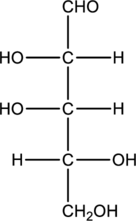
Concept Introduction:
Monosaccharides that are aldoses contains an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Principles of General Organic & Biological Chemistry
Ch. 14.1 - Draw a Lewis structure for glucose that clearly...Ch. 14.2 - Prob. 14.2PCh. 14.2 - Prob. 14.3PCh. 14.2 - Prob. 14.4PCh. 14.2 - Rank the following compounds in order of...Ch. 14.2 - Prob. 14.6PCh. 14.2 - Prob. 14.7PCh. 14.2 - Prob. 14.8PCh. 14.2 - Prob. 14.9PCh. 14.3 - Prob. 14.10P
Ch. 14.3 - Prob. 14.11PCh. 14.4 - Prob. 14.12PCh. 14.4 - Prob. 14.13PCh. 14.4 - Prob. 14.14PCh. 14.4 - Prob. 14.15PCh. 14.5 - Prob. 14.16PCh. 14.5 - Prob. 14.17PCh. 14.5 - Prob. 14.18PCh. 14.5 - Prob. 14.19PCh. 14.6 - Prob. 14.20PCh. 14 - Prob. 14.21UKCCh. 14 - Prob. 14.22UKCCh. 14 - Prob. 14.23UKCCh. 14 - Prob. 14.24UKCCh. 14 - Prob. 14.25UKCCh. 14 - Prob. 14.26UKCCh. 14 - Prob. 14.27UKCCh. 14 - Prob. 14.28UKCCh. 14 - Prob. 14.29UKCCh. 14 - Prob. 14.30UKCCh. 14 - Prob. 14.31APCh. 14 - Prob. 14.32APCh. 14 - Prob. 14.33APCh. 14 - Prob. 14.34APCh. 14 - Prob. 14.35APCh. 14 - Prob. 14.36APCh. 14 - Prob. 14.37APCh. 14 - Prob. 14.38APCh. 14 - Prob. 14.39APCh. 14 - Prob. 14.40APCh. 14 - Prob. 14.41APCh. 14 - Prob. 14.42APCh. 14 - Prob. 14.43APCh. 14 - Prob. 14.44APCh. 14 - Prob. 14.45APCh. 14 - Prob. 14.46APCh. 14 - Prob. 14.47APCh. 14 - Prob. 14.48APCh. 14 - Prob. 14.49APCh. 14 - Prob. 14.50APCh. 14 - Prob. 14.51APCh. 14 - Prob. 14.52APCh. 14 - Prob. 14.53APCh. 14 - Prob. 14.54APCh. 14 - Prob. 14.55APCh. 14 - Prob. 14.56APCh. 14 - Prob. 14.57APCh. 14 - Prob. 14.58APCh. 14 - Prob. 14.59APCh. 14 - Prob. 14.60APCh. 14 - Prob. 14.61APCh. 14 - Prob. 14.62APCh. 14 - Prob. 14.63APCh. 14 - Prob. 14.64APCh. 14 - Prob. 14.65APCh. 14 - Prob. 14.66APCh. 14 - Prob. 14.67APCh. 14 - Prob. 14.68APCh. 14 - Prob. 14.69APCh. 14 - Prob. 14.70APCh. 14 - Prob. 14.71APCh. 14 - Prob. 14.72APCh. 14 - Prob. 14.73CPCh. 14 - Prob. 14.74CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY