
Interpretation:
The cubane,
Concept introduction:
The complex vibrations exhibit by the polyatomic molecule is known as normal modes of vibrations. The vibrational modes of a molecule are IR or Raman active. If a molecule has centre of symmetry, then the modes which are IR-active will be Raman inactive and the modes that are IR-inactive will be Raman active. The total number of vibrational degrees of freedom for nonlinear molecule is represented by
Answer to Problem 14.82E
The cubane,
Explanation of Solution
The structure of cubane is shown below.
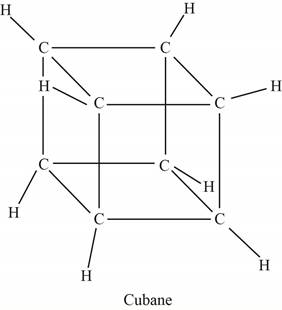
Figure 1
The point group of cubane is
The character table for point group
The expression
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The expression
Substitute the value of
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The great orthogonality theorem for the reducible representation can be represented as,
Where,
•
•
•
•
•
The term
The irreducible representation for point group
Substitute the
Therefore, the cubane,
The cubane,
Want to see more full solutions like this?
Chapter 14 Solutions
Physical Chemistry
- Determine the number of total degrees of freedom and the number of vibrational degrees of freedom for the following species. a Hydrogen sulfide, H2S b Carbonyl sulfide, OCS c The sulfate ion, SO42 d Phosgene, COCl2 e Elemental chlorine, Cl2 f A linear molecule having 20 atoms g A nonlinear molecule having 20 atomsarrow_forwardWhich of the following molecules should have pure rotational spectra? a Dimethyltriacetylene, H3CCCCCCCCH3 b Cyanotetraacetylene, HCCCCCCCCCN Such molecules have been detected in intersteller space. c Nitric oxide, NO d Nitrogen dioxide, NO2 e Sulfur tetrafluoride, SF4 f Sulfur hexafluoride, SF6arrow_forwardWhat is the most highly populated rotational level of Br2 at (i) 25 °C, (ii) 100 °C? Take ᷉ B = 0.0809 cm−1.arrow_forward
- The hydrogen halides have the following fundamental vibrational wavenumbers: 4141.3 cm−1 (1H19F); 2988.9 cm−1 (1H35Cl); 2649.7 cm−1 (1H81Br); 2309.5 cm−1 (H127I). Calculate the force constants of the hydrogen–halogen bonds.arrow_forwardCalculate the isotope shift in the fundamental vibrational frequencies of 2H79Br,1H81 with respect to1H79 if the value of force constant is 380 cm-1 for all the three isotopes. -2 cm-1,-816 cm-1 -2 cm-1,-750 cm-1 0 cm-1 ,-620 cm-1 0 cm-1 ,-731 cm-1arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

