
Interpretation:
The mass of mercury in grams contained in each
Concept introduction:
Meter square
Mass is a physical quantity and its SI unit is kilograms
The conversion of one unit into another can be done using a proper conversion factor. Conversion factors are the ratios that relate the two different units of a quantity. It is also known as dimensional analysis or factor label method.
In the unit conversion problems, the given information is multiplied by the conversion factors to obtain the desired information. The unit conversion can be done as follows:
Answer to Problem 1.6BFP
The road map to calculate the mass of mercury in grams contained in each
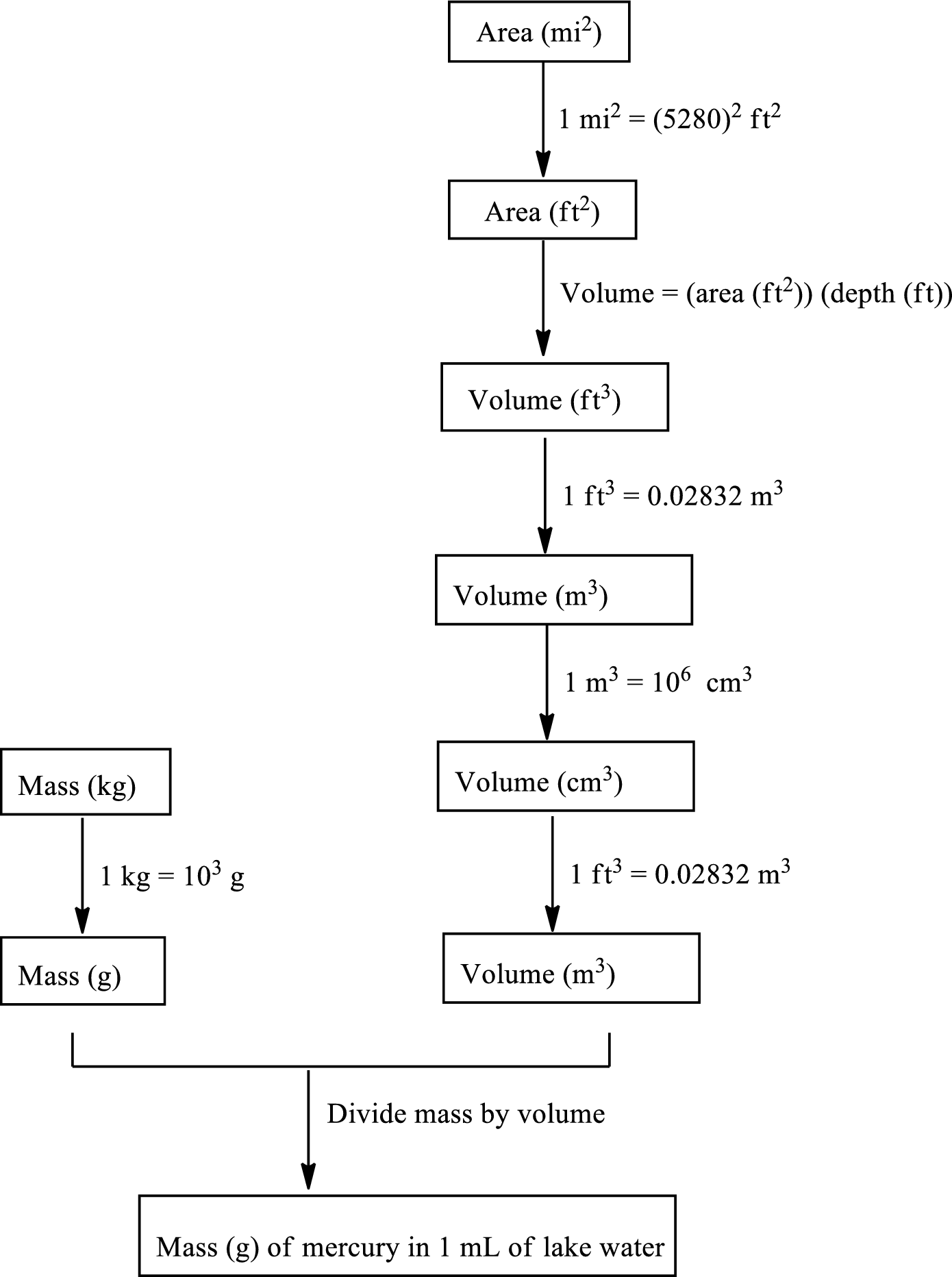
The mass of mercury in grams contained in each
Explanation of Solution
The road map to calculate the mass of mercury in grams contained in each
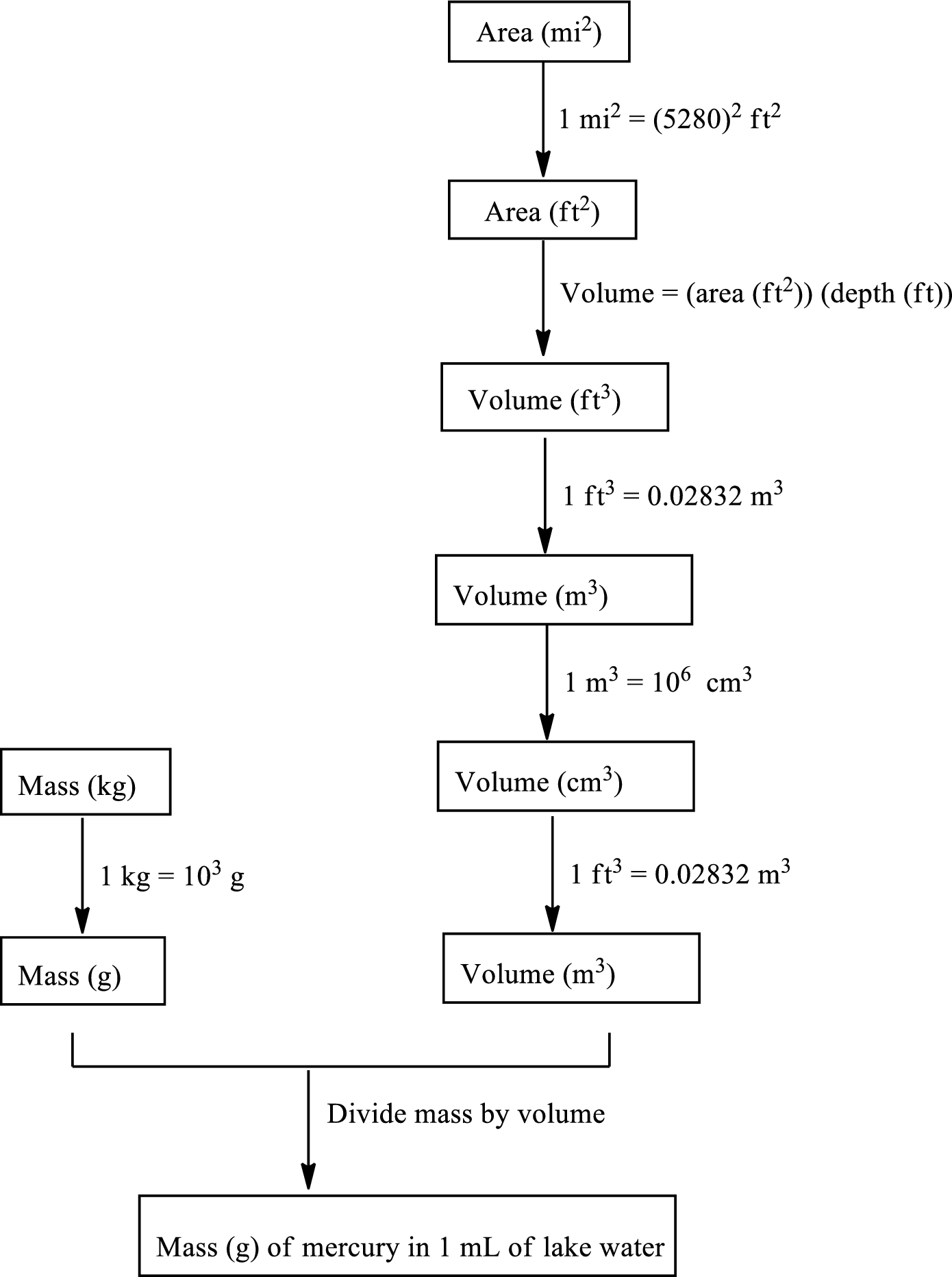
The mass of mercury discharged in the lake is
The conversion factor to convert the mass from kilograms
The mass of mercury in the lake in grams is,
The surface area of the lake is
The conversion factor to convert the area from
The surface area of the lake in
The volume of the lake is calculated as:
Substitute
The conversion factor to convert volume from
The conversion factor to convert volume from
The conversion factor to convert volume from
The volume of the lake in
The mass of mercury in grams present in one
Substitute
The mass of mercury in grams contained in each
Want to see more full solutions like this?
Chapter 1 Solutions
Chemistry: The Molecular Nature of Matter and Change
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





