
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
10th Edition
ISBN: 9781260001099
Author: Carey
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 36P
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
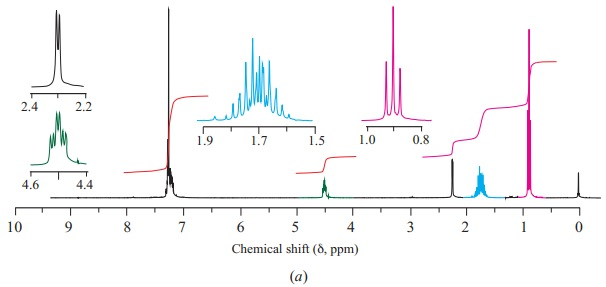
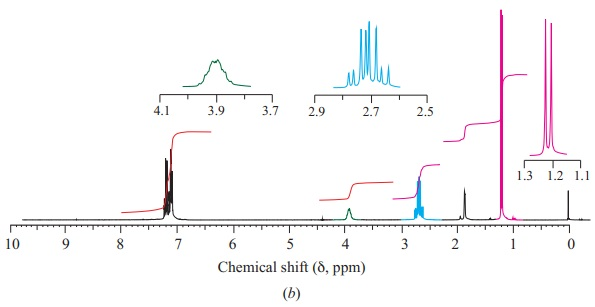
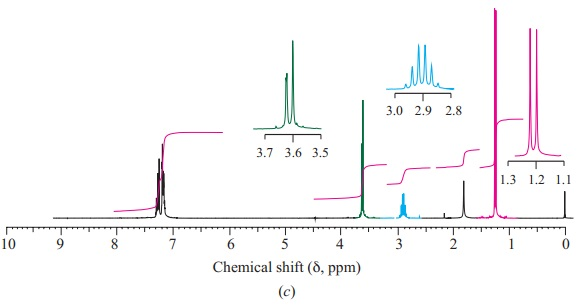
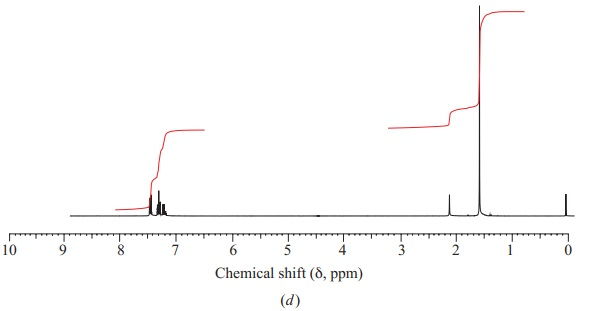
Show which carbon atoms correspond with which peaks in the 13C NMR spectrum ofbutan-2-one
These are four alcohols with the formula C4H10O. One of them produced this C-NMR spectrum, which alcohol did that and what carbons correspond with what peaks?
1. Nomex is a flame resistant polymer developed by DuPont in the 1960s. Itis made by polymerizing benzene-1,3-diamine and isophthaloyl chloride.
a) Draw three repeating units of Nomex.
b) How many peaks will there be in the 1 H NMR spectrum of Nomex,assuming a polymer strand is infinitely long?
c) How many peaks will there be in the 13 C NMR spectrum of Nomex,assuming a polymer strand is infinitely long?
Chapter 14 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
Ch. 14.3 - Prob. 1PCh. 14.3 - Prob. 2PCh. 14.4 - The 1H NMR signal for bromoform (CHBr3) appears at...Ch. 14.5 - identify the most shielded and least shielded...Ch. 14.5 - (a) Assign the chemical shifts 1.6, 2.2, and 4.8...Ch. 14.5 - Assign the chemical shifts 1.1, 1.7, 2.0, and 2.3...Ch. 14.5 - Assign the chemical shifts 1.6, 4.0, 7.5, 8.2, and...Ch. 14.6 - The 300-MHz 1H NMR spectrum of 1,4-dimethylbenzene...Ch. 14.6 - Prob. 9PCh. 14.6 - How many signals would you expect to find in the...
Ch. 14.7 - Describe the appearance of the 1H NMR spectrum of...Ch. 14.8 - Describe the appearance of the 1H NMR spectrum of...Ch. 14.11 - Prob. 13PCh. 14.11 - Prob. 14PCh. 14.12 - Hydrogen bonding between the oxygen of dimethyl...Ch. 14.14 - Prob. 16PCh. 14.15 - The 13C NMR spectrum of 1-bromo-3-chloropropane...Ch. 14.15 - Consider carbons x, y, and z in p-methylanisole....Ch. 14.15 - Prob. 19PCh. 14.16 - To which of the compounds of Problem 14.16 does...Ch. 14.18 - DEPT spectra for a compound with the formula...Ch. 14.20 - Vibrational frequencies are sensitive to isotopic...Ch. 14.21 - Prob. 23PCh. 14.22 - Prob. 24PCh. 14.23 - Prob. 25PCh. 14.23 - Which one of the C5H8 isomers shown has its max at...Ch. 14.24 - Knowing what to look for with respect to isotopic...Ch. 14.24 - The base peak appears at m/z105 for one of the...Ch. 14.24 - Mass spectra of 1-bromo-4-propylbenzene and...Ch. 14.25 - Prob. 30PCh. 14 - Each of the following compounds is characterized...Ch. 14 - Deduce the structure of each of the following...Ch. 14 - From among the isomeric compounds of molecular...Ch. 14 - The H1NMR spectrum of fluorene has signals at 3.8...Ch. 14 - Prob. 35PCh. 14 - H1NMR spectra of four isomeric alcohols with...Ch. 14 - Prob. 37PCh. 14 - We noted in Section 14.13 that an NMR spectrum is...Ch. 14 - Identify each of the C4H10O isomers on the basis...Ch. 14 - A compound (C3H7ClO2) exhibited three peaks in its...Ch. 14 - Label nonequivalent carbons in the following...Ch. 14 - Compounds A and B are isomers of molecular formula...Ch. 14 - C13 NMR spectra for four isomeric alkyl bromides...Ch. 14 - Prob. 44PCh. 14 - Prob. 45PCh. 14 - Identify the C3H5Br isomers on the basis of the...Ch. 14 - Prob. 47PCh. 14 - A compound (C8H10O) has the IR and H1NMR spectra...Ch. 14 - Deduce the structure of a compound having the...Ch. 14 - Figure 14.53 presents IR, H1NMR, C13NMR and mass...Ch. 14 - H1NMR, C13NMR, IR, and mass spectra are shown for...Ch. 14 - 1H NMR and IR spectra for a compound with the...Ch. 14 - FriedelCraftsalkylation of benzene with...Ch. 14 - Prob. 54DSPCh. 14 - Prob. 55DSPCh. 14 - Prob. 56DSPCh. 14 - Prob. 57DSPCh. 14 - Prob. 58DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- can you list the corresponding ppm values for the non-aromatic carbon atom in ^13C NMR spectrum for benzonitrile?arrow_forwardwhat is the structure for this H NMR, C5H9BrO2 HDI = 1arrow_forwardThe following 1H NMR peaks were recorded on a spectrometer operating at 200 MHz. Convert each into δ units. (a) CHCl3; 1454 Hz (b) CH3Cl; 610 Hz (c) CH3OH; 693 Hz (d) CH2Cl2; 1060 Hzarrow_forward
- The mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectral data.arrow_forwardThe 13C-NMR spectrum of 3-methyl-2-butanol shows signals at 17.88 (CH3), 18.16 (CH3), 20.01 (CH3), 35.04 (carbon-3), and 72.75 (carbon-2). Account for the fact that each methyl group in this molecule gives a different signal.arrow_forwardPropose structures for compounds that fit the following descriptions: (a) A hydrocarbon with seven lines in its 13C NMR spectrum (b) A six-carbon compound with only five lines in its 13C NMR spectrum (c) A four-carbon compound with three lines in its 13C NMR spectrumarrow_forward
- 3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which the vinylic proton at C2 is coupled with both the C1 vinylic proton (J = 16 Hz) and the C3 methylene protons (J = 8 Hz). Draw a tree diagram for the C2 proton signal, and account for the fact that a five-line multiplet is observed.arrow_forward3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 . Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be helpful.arrow_forwardAscaridole is a natural product that has been used to treat intestinal worms. Explain why the two methyls on the isopropyl group in ascaridole appear in its 1H-NMR spectrum as four lines of equal intensity, with two sets of two each separated by 7 Hz.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY