
Concept explainers
NMR spectra for four isomeric alkyl bromides with the formula
Assign structures to each of the alkyl bromides and assign the peaks in each spectrum.
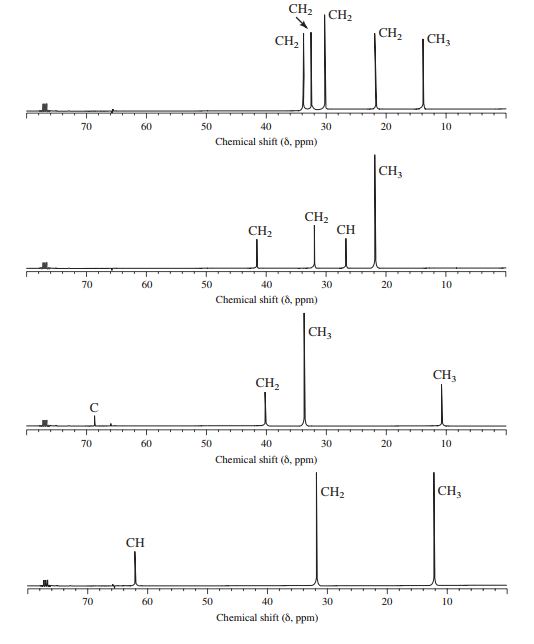
Interpretation:
Structures of each of the isomeric alkyl bromides with the formula
Concept introduction:
In
Carbon atoms having equivalent environment will have the same chemical shift.
In simple molecules, equivalent carbon atoms can be identified just by inspection.
The number of signals depends on the number of distinct carbon atoms in the structure of the compound.
If the structure is symmetrical, the number of signals in the
The electronegative atom/group decreases the shielding of the carbon atom to which it is attached.
In
An
The chemical shift for methyl carbon atoms ranges from
The chemical shift for methylene carbon atoms ranges from
The chemical shift for methine carbon atoms ranges from
The chemical shift for aromatic carbon atoms ranges from
The index of hydrogen deficiency is calculated as follows:
Here
Answer to Problem 43P
Solution:
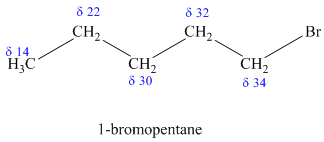
a)
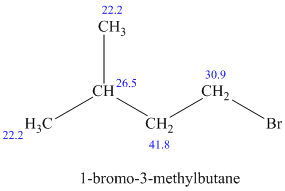
b)
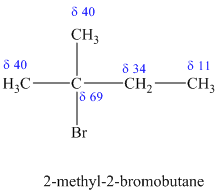
c)
d)
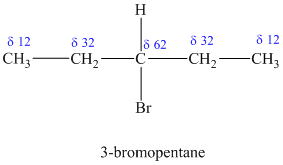
Explanation of Solution
a)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
Combining all this, the only possible structure for the isomer having the formula

b)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
The signal at
Combining all this, the only possible structure for the isomer having the formula
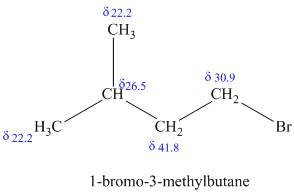
c)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
The signal at
The chemical shift values for the remaining two signals indicate two types of methyl groups. The signal at
Combining all this, the only possible structure for the isomer having the formula

d)
The molecular formula shows an index of hydrogen deficiency equal to zero. Thus, the compound contains ring or multiple bonds. All the carbon atoms must be
The signal at
The signal at
Combining all this, the only possible structure for the isomer having the formula

Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- How many peaks would you expect in the 1H NMR spectrum of 1, 4-dimethyl- benzene (para-xylene, or p-xylene)? What ratio of peak areas would you expect on integration of the spectrum? Refer to Table 13-3 for approximate chemical shifts, and sketch what the spectrum would look like. (Remember from Section 2-4 that aromatic rings have two resonance forms.)arrow_forward3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which the vinylic proton at C2 is coupled with both the C1 vinylic proton (J = 16 Hz) and the C3 methylene protons (J = 8 Hz). Draw a tree diagram for the C2 proton signal, and account for the fact that a five-line multiplet is observed.arrow_forwardThe mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectral data.arrow_forward
- The 1H and 13C NMR spectra of compound A, C8H9Br, are shown. Propose a structure for A, and assign peaks in the spectra to your structure.arrow_forward3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 . Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be helpful.arrow_forwardTreatment of 3,4-dibromohexane with strong base leads to loss of 2 equivalents of HBr and formation of a product with formula C6H10. Three products are possible. Name each of the three, and tell how you would use 1H and 13CNMR spectroscopy to help identify them. How would you use UV spectroscopy?arrow_forward
- When measured on a spectrometer operating at 200 MHz, chloroform (CHCl3) shows a single sharp absorption at 7.3 δ. (a) How many parts per million downfield from TMS does chloroform absorb? (b) How many hertz downfield from TMS would chloroform absorb if the measurement were carried out on a spectrometer operating at 360 MHz? (c) What would be the position of the chloroform absorption in δ units when measured on a 360 MHz spectrometer?arrow_forwardWhen the 1HNMR spectrum of an alcohol is run in dimethylsulfoxide (DMSO) solvent rather than in chloroform, exchange of the Ο-H proton is slow and spin-spin splitting is seen between the Ο-H proton and C-H protons on the adjacent carbon. What spin multiplicities would you expect for the hydroxyl protons in the following alcohols? (a) 2-Methyl-2-propanol (b) Cyclohexanol (c) Ethanol (d) 2-Propanol (e) Cholesterol (f) 1-Methylcyclohexanolarrow_forwardHow many peaks would you expect to see in the 13C NMR spectrum of 3-bromohexane? a.) 3 b.) 4 c.) 5 d.) 6arrow_forward
- Where do you get the O-H spectroscopy of 3200-3400 & the O-C of 1200-1300?arrow_forwardFor each compound shown below,(1) sketch the 13C NMR spectrum (totally decoupled, with a singlet for each type of carbon), showing approximatechemical shifts.(2) show the multiplicity expected for each signal in the off-resonance-decoupled spectrum.(3) sketch the spectra expected using the DEPT-90 and DEPT-135 techniques.(a)OCH3 C O CH2 CH3ethyl acetatearrow_forwardHow many peaks do you expect in the 13C NMR spectrum of the product?arrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



