
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 4PP
Practice Problem 14.4
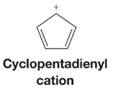
Apply the polygon-and-circle method to the cyclopentadienyl cation and explain whether it would be aromatic or not.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A student was given from the list of the compounds below A, B and D blindly and asked to identify them all. He treated each of them with Brady's reagent (2,4-ditrophenylhydrazine) and isolated a bright yellow compound for one of them, but the other two gave false negatives. The student reasoned that the false negatives may be due to sterics and, on further thinking, it dawned on him that he might be able to rule out one of the false negatives with the haloform test. What compound did he find compatible with the haloform test? That compound did indeed give a false negative in the Brady test. Which of the other two was positive in the Brady test?
A = haloform
B = Brady
A = haloform
D = Brady
B = haloform
A = Brady
B = haloform
D = Brady
D = haloform
A = Brady
D = haloform
B = Brady
When the nitrogen-containing aromatic heterocyclic compounds 1 and 2 are treated with HCl, only 1 forms the hydrochloride salt, whereas compound 2 is unreactive. Provide an explanation for this observed reactivity.
Using the Frost Circle method to outline the molecular orbitals of cyclobutadiene, and identify whether it is aromatic, antiaromatic or non-aromatic. Explain.
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - PRACTICE PROBLEM 14.1 Provide a name for each of...Ch. 14 - Prob. 2PPCh. 14 - Prob. 3PPCh. 14 - Practice Problem 14.4 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.5 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.6 1,3,5-Cycloheptatriene is...Ch. 14 - Prob. 7PPCh. 14 - Prob. 8PPCh. 14 - Practice Problem 14.9 In 1967 R. Breslow (of...Ch. 14 - Prob. 10PP
Ch. 14 - Practice Problem 14.11 In addition to a signal...Ch. 14 - PRACTICE PROBLEM 14.12
Azulene has an appreciable...Ch. 14 - Practice Problem 14.13 (a) The -Sh group is...Ch. 14 - Practice Problem 14.14
Explain how NMR...Ch. 14 - PRACTICE PROBLEM 14.15 Four benzenoid compounds,...Ch. 14 - Prob. 16PCh. 14 - Write structural formulas and give acceptable...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Which of the hydrogen atoms shown below is more...Ch. 14 - 14.22 The rings below are joined by a double bond...Ch. 14 - Prob. 23PCh. 14 - 14.24 (a) In 1960 T. Katz (Columbia University)...Ch. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - 14.27 5-Chloro-1,3-cyclopentadiene (below)...Ch. 14 - Prob. 28PCh. 14 - Furan possesses less aromatic character than...Ch. 14 - 14.30 For each of the pairs below, predict...Ch. 14 - Assign structures to each of the compounds A, B,...Ch. 14 - Prob. 32PCh. 14 - Give a structure for compound F that is consistent...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - The IR and 1H NMR spectra for compound X(C8H10)...Ch. 14 - Prob. 37PCh. 14 - Prob. 38PCh. 14 - 14.39 Given the following information, predict the...Ch. 14 - Consider these reactions: The intermediate A is a...Ch. 14 - Prob. 41PCh. 14 - Compound E has the spectral features given below....Ch. 14 - Draw all of the molecular orbitals for...Ch. 14 - Prob. 1LGPCh. 14 - Prob. 2LGPCh. 14 - 3. The NMR signals for the aromatic hydrogens of...Ch. 14 - Prob. 4LGPCh. 14 - Prob. 5LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
65. Determine the molecular geometry of each molecule.
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
fill in the reagents you would use for the following transformations:
Organic Chemistry As a Second Language: Second Semester Topics
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
23. Give the symbol and name for (a) an isotope with a mass number of 37 and an atomic number of 17 and (b) an ...
Chemistry For Changing Times (14th Edition)
The type of organic compound which is nepetalactone needs to be determined. Concept introduction: The type of a...
Chemistry: Matter and Change
Calculate the quantity of heat, in kilojoules, (a) required to raise the temperature of 9.25 L of water from 22...
General Chemistry: Principles and Modern Applications (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- write a mechanism for the Friedel-Crafts reaction of anisole with acetic anhydride to produce what you believe is the major isomer of methoxyacetophenone. Be sure to show all structures of reactants, products and intermediates as well as the movement of electrons in each step.arrow_forwardGive the structure of compound 1 and compound 2arrow_forwardBringing together your knowledge of the reaction chemistry of substituted benzenes, suggest a preparative route for conversion of compound A to compound B shown belowarrow_forward
- 1. If diene is used in excess for a Diels-alder reaction of a-phellandrene and malice acid, which side reaction would be expected? Use chemical equations to support your answer. 2. If methanol was used instead of diethyl ether as the reaction solvent and left tp reflux longer than necessary, what side reactions would you expect to happen. in the reaction flask. Use a chemical equation to support your answer.arrow_forward1. Predict the maximum total number of possible peaks in the 13C NMR spectrum of sesamin (Hint: , keep in mind the molecule’s symmetry when predicting the # of NMR peaks) a. Indicate the number of non-aromatic peaks in the 13C NMR of sesamin b.Indicate the number of aromatic peaks in the 13C NMR of sesaminarrow_forwardCompound A produce compound D while undergo Friedel Crafts Alkylation. Compound D is then oxidized and produce compound E (C11H12O3) as a major product.What are the possible structural formula of compound D and E?arrow_forward
- Starting with acid chloride with exactly 5 carbon atoms, and using appropriate reagents outline the synthesis of the following molecules:arrow_forwardOutline a synthesis for the following transformation and provide a justification for your chosen strategy. (More than one steps may be required)arrow_forwardProvide a mechanistic explanation for the following observation.arrow_forward
- A certain compound is known to contain an aromatic benzene ring but failed to produce a fragrant yellow solution upon subjecting it to the nitration test. What may be a possible explanation for this? A. The benzene ring is part of a highly conjugated, blue dye molecule. B. The benzene ring contains a strong electron-withdrawing group. C. The benzene ring has no available sites left for electrophilic attack. D. All of the given. Kindly explain your answer in detail.arrow_forwardDraw the structure of the desired dibromide product H. ) Propose a reasonable arrow-pushing mechanism for formation of the byproduct I.Use your mechanism to justify the stereochemistry in molecule Iarrow_forwardIdentify the structure of the compound a with a formula of C3H6O2 having the following IR, and 1H NMR spectra (integrals and multiplicity shown in the boxes: s-singlet, d-doublet, t-triplet, q-quartet, etc.). Label the spectra with proper information you deduced (functional groups, number of protons, fragments, etc.). Compound formula. C3H6O2 Questions to help you decide What is the element of unsaturation of the molecular formula? Show the equation you use: What are the functional groups present in this molecule? Show all of them belowDraw at least two possible structures that have the . required element of unsaturation as well as the observed functional groups: Based on the 1H NMR above, what molecular fragmentations do you see: Draw your final decision of the structure below. Is this one of the structures in your answer 5C?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY