
Organic Chemistry, Books A La Carte Edition (7th Edition)
7th Edition
ISBN: 9780321819031
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 64P
Give approximate wavenumbers for the major characteristic IR absorption bands that would be given by each of the following compounds:
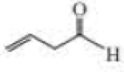
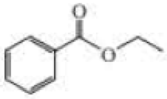
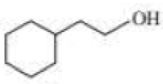
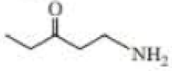
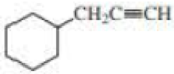
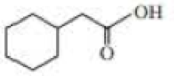
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
which of the following compounds present bands in the IR spectrum at 3000-3500 cm-1, 1750 cm-1 and 1220 cm-1.
1. True or False
a. UV-Vis spectroscopy normally reports data in the from of bands rather than single peaks because of overlapping electronic transitions that are being recorded by the detector. (T/F)
b. When using linear regression to translate absorption data using Beer's Law, the y-intercept (+b) of the linear equation represents the path length. (T/F)
c. Phosphorescent materials give a glowing effect because the electrons remain at an excited state for much longer, which is the cause for the "glow". (T/F)
For each of the following pairs of compounds, name one absorption band that can be used to distinguish between them. State what the bond is (bond order, between two atoms) and approximately where it appears in the IR spectrum (in cm-1)
Chapter 14 Solutions
Organic Chemistry, Books A La Carte Edition (7th Edition)
Ch. 14.1 - Which of the following fragments produced in a...Ch. 14.2 - What distinguishes the mass spectrum of...Ch. 14.2 - What is the most likely m/z value for the base...Ch. 14.3 - Prob. 5PCh. 14.3 - If a compound has a molecular ion with an...Ch. 14.3 - a. Suggest possible molecular formulas for a...Ch. 14.3 - Identify the hydrocarbon that has a molecular ion...Ch. 14.4 - Predict the relative intensities of the molecular...Ch. 14.5 - Which molecular formula has an exact molecular...Ch. 14.5 - Prob. 11P
Ch. 14.6 - Sketch the mass spectrum expected for...Ch. 14.6 - The mass spectra of 1-methoxybutane,...Ch. 14.6 - Prob. 14PCh. 14.6 - Identify the ketones responsible for the mass...Ch. 14.6 - Prob. 16PCh. 14.6 - Using curved arrows, show the principal fragments...Ch. 14.6 - The reaction of (Z)-2-pentene with water and a...Ch. 14.9 - Prob. 19PCh. 14.9 - Prob. 20PCh. 14.9 - Prob. 21PCh. 14.13 - Prob. 22PCh. 14.14 - Which occur at a larger wavenumber: a. the C O...Ch. 14.14 - Prob. 24PCh. 14.14 - Prob. 25PCh. 14.14 - Rank the following compounds from highest...Ch. 14.14 - Which shows an O H stretch at a larger...Ch. 14.15 - Prob. 28PCh. 14.15 - a. An oxygen-containing compound shows an...Ch. 14.15 - Prob. 30PCh. 14.15 - For each of the following pair of compounds, name...Ch. 14.16 - Which of the following compounds has a vibration...Ch. 14.16 - Prob. 33PCh. 14.17 - A compound with molecular formula C4H6O gives the...Ch. 14.19 - Prob. 35PCh. 14.19 - Prob. 36PCh. 14.20 - Predict the max of the following compound:Ch. 14.20 - Prob. 38PCh. 14.21 - a. At pH = 7 one of the ions shown here is purple...Ch. 14.21 - Prob. 40PCh. 14.22 - Prob. 41PCh. 14.22 - Prob. 42PCh. 14 - In the mass spectrum of the following compounds,...Ch. 14 - Prob. 44PCh. 14 - For each of the following pairs of compounds,...Ch. 14 - Draw structures for a saturated hydrocarbon that...Ch. 14 - a. How could you use IR spectroscopy to determine...Ch. 14 - Assuming that the force constant is approximately...Ch. 14 - In the following boxes, list the types of bonds...Ch. 14 - A mass spectrum shows significant peaks at m/z. =...Ch. 14 - Prob. 51PCh. 14 - Prob. 52PCh. 14 - Prob. 53PCh. 14 - How can you use UV spectroscopy to distinguish...Ch. 14 - Rank the following compounds from highest...Ch. 14 - Rank the following compounds from highest...Ch. 14 - What peaks in their mass spectra can be used to...Ch. 14 - Each of the IR spectra shown below is accompanied...Ch. 14 - Prob. 59PCh. 14 - Prob. 60PCh. 14 - How can IR spectroscopy distinguish between...Ch. 14 - 62. Draw the structure of a carboxylic acid that...Ch. 14 - Prob. 63PCh. 14 - Give approximate wavenumbers for the major...Ch. 14 - Prob. 65PCh. 14 - Prob. 66PCh. 14 - Prob. 67PCh. 14 - The IR spectrum of a compound with molecular...Ch. 14 - Which one of the following live compounds produced...Ch. 14 - Prob. 70PCh. 14 - Phenolphthalein is an acid-base indicator. In...Ch. 14 - Which one of the following five compounds produced...Ch. 14 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following compounds will show two absorption bands at around 3300 cm-1 and around 2150 cm-¹?arrow_forwardDescribe the vibrational frequencies for the carbonyl bands of IR spectra for the following compounds: 1. Acetophenone 2. Benzaldehyde 3. Benzamide 4. Benzoic acid 5. Benzoyl Chloride 6. Methyl Benzoate Also, list the order of decreasing carbonyl frequency for the above compounds.arrow_forwardFind the major IR Absorption bands and give a proposed molecular compositionarrow_forward
- Among the following molecules, identify the one that corresponds to the IR spectrum presented below. Explain your choice by indicating the significant bands with their approximate wavenumber valuesarrow_forwardExplain why a basic solution of methyl ethylamine is neutralized by the addition of a concentrated hydrochloric acid, its weak absorption band centered at 244 nm starts to disappeararrow_forwardWhich of the following molecules best describes the IR spectrum given with a molecular formula of C6H12O2?arrow_forward
- The IR and 1H NMR spectra of an unknown molecular formula C8H15NO2 are shown below. The following questions will guide you in determining its structure. 1.Determine the degree of unsaturation. Show your calculation.2.Analyze three significant bands in the IR spectra and conclude on the existing functions.3.Analyze the 1H NMR chart below Draw the final structure of the unknownarrow_forwardDiscuss every labelled peak in the IR spectrum and identify functional group using the provided table 15.2 (Wavenumbers).arrow_forwardAt what approximate positions might the following compounds show IR absorptions?arrow_forward
- Do the following steps for the unknown C3H7Br Determine the Saturation Index Identify IR and NMR bands Determine and draw the stucture of the unknownarrow_forwardExplain the following trend in the absorptions corresponding to the most energetic C–H stretching mode observed in the IR spectra of the following molecules.arrow_forward1. Using the following on the positions of peaks in IR spectra and empirical formulas, propose possible structures for the molecules. Express all isomers that match given spectra. Explain the choices. A. C5O2H8 : 3010, 2960, 1650, 1740, 1030, 1430, and 1380 (cm-1 ) B. C8OH8 : 1600, 1680, 2960, 1480, 1380, 755, and 690 (cm-1 ) C. C4H5N : 1650, 2250, 1480, 3010, and 2960 (cm-1 )arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY