
Organic Chemistry, Books A La Carte Edition (7th Edition)
7th Edition
ISBN: 9780321819031
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 45P
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them:

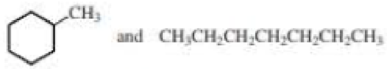
- a. CH3CH2CH2OH and CH3CH3OCH3


- b. cis-2-butene and trans-2-butene

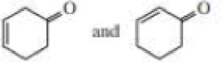
- c. i CH3CH2CH=CHCH3 and CH3CH2C=CCH3

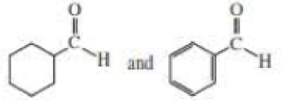

NOTE TO THE STUDENT
• There are additional spectroscopy problems in the Study Guide and Solution Manual.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Write the IR spectra to the corresponding structures below and cite the applicable peaks
A. Heptanol
B. Acetanilide
1. which spectroscopy tool would be the best to distinguish a sample of 1,2,2-trichloropropane from 1,1,2-triclopropane a) HNMR b) mass spectrometry c) infrared spectroscopy
1. Using the following on the positions of peaks in IR spectra and empirical formulas, propose possible structures for the molecules. Express all isomers that match given spectra. Explain the choices.
A. C5O2H8 : 3010, 2960, 1650, 1740, 1030, 1430, and 1380 (cm-1 )
B. C8OH8 : 1600, 1680, 2960, 1480, 1380, 755, and 690 (cm-1 )
C. C4H5N : 1650, 2250, 1480, 3010, and 2960 (cm-1 )
Chapter 14 Solutions
Organic Chemistry, Books A La Carte Edition (7th Edition)
Ch. 14.1 - Which of the following fragments produced in a...Ch. 14.2 - What distinguishes the mass spectrum of...Ch. 14.2 - What is the most likely m/z value for the base...Ch. 14.3 - Prob. 5PCh. 14.3 - If a compound has a molecular ion with an...Ch. 14.3 - a. Suggest possible molecular formulas for a...Ch. 14.3 - Identify the hydrocarbon that has a molecular ion...Ch. 14.4 - Predict the relative intensities of the molecular...Ch. 14.5 - Which molecular formula has an exact molecular...Ch. 14.5 - Prob. 11P
Ch. 14.6 - Sketch the mass spectrum expected for...Ch. 14.6 - The mass spectra of 1-methoxybutane,...Ch. 14.6 - Prob. 14PCh. 14.6 - Identify the ketones responsible for the mass...Ch. 14.6 - Prob. 16PCh. 14.6 - Using curved arrows, show the principal fragments...Ch. 14.6 - The reaction of (Z)-2-pentene with water and a...Ch. 14.9 - Prob. 19PCh. 14.9 - Prob. 20PCh. 14.9 - Prob. 21PCh. 14.13 - Prob. 22PCh. 14.14 - Which occur at a larger wavenumber: a. the C O...Ch. 14.14 - Prob. 24PCh. 14.14 - Prob. 25PCh. 14.14 - Rank the following compounds from highest...Ch. 14.14 - Which shows an O H stretch at a larger...Ch. 14.15 - Prob. 28PCh. 14.15 - a. An oxygen-containing compound shows an...Ch. 14.15 - Prob. 30PCh. 14.15 - For each of the following pair of compounds, name...Ch. 14.16 - Which of the following compounds has a vibration...Ch. 14.16 - Prob. 33PCh. 14.17 - A compound with molecular formula C4H6O gives the...Ch. 14.19 - Prob. 35PCh. 14.19 - Prob. 36PCh. 14.20 - Predict the max of the following compound:Ch. 14.20 - Prob. 38PCh. 14.21 - a. At pH = 7 one of the ions shown here is purple...Ch. 14.21 - Prob. 40PCh. 14.22 - Prob. 41PCh. 14.22 - Prob. 42PCh. 14 - In the mass spectrum of the following compounds,...Ch. 14 - Prob. 44PCh. 14 - For each of the following pairs of compounds,...Ch. 14 - Draw structures for a saturated hydrocarbon that...Ch. 14 - a. How could you use IR spectroscopy to determine...Ch. 14 - Assuming that the force constant is approximately...Ch. 14 - In the following boxes, list the types of bonds...Ch. 14 - A mass spectrum shows significant peaks at m/z. =...Ch. 14 - Prob. 51PCh. 14 - Prob. 52PCh. 14 - Prob. 53PCh. 14 - How can you use UV spectroscopy to distinguish...Ch. 14 - Rank the following compounds from highest...Ch. 14 - Rank the following compounds from highest...Ch. 14 - What peaks in their mass spectra can be used to...Ch. 14 - Each of the IR spectra shown below is accompanied...Ch. 14 - Prob. 59PCh. 14 - Prob. 60PCh. 14 - How can IR spectroscopy distinguish between...Ch. 14 - 62. Draw the structure of a carboxylic acid that...Ch. 14 - Prob. 63PCh. 14 - Give approximate wavenumbers for the major...Ch. 14 - Prob. 65PCh. 14 - Prob. 66PCh. 14 - Prob. 67PCh. 14 - The IR spectrum of a compound with molecular...Ch. 14 - Which one of the following live compounds produced...Ch. 14 - Prob. 70PCh. 14 - Phenolphthalein is an acid-base indicator. In...Ch. 14 - Which one of the following five compounds produced...Ch. 14 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify all the peaks from the IR spectrum. Be sure to list the cm-1 and the bond that corresponds to each peak.arrow_forwardWhat are the important diagnostic peaks in this IR spectra? Fingerprint region <1500cm^-1 is not necessary. Thank you.arrow_forwardHere are proton NMR data for 1-bromopropane: Ha : triplet (2H) 3.32ppm; Hb : multiplet (2H)1.81ppm; Hc : triplet (3H) 0.93ppm. (Relative integrations shown in parentheses.) a. Through how many bonds can a hydrogen split another hydrogen? b. According to this splitting rule, does Ha split Hc ? c. Is your answer in part a) consistent with the multiplicity listed for peak clusters a and c? d. How many hydrogenssplit Hb ? e. Upon very close inspection of the proton NMR spectrum of 1-bromopropane, you wouldfind that peak cluster b has at least six peaks. Is this consistent with your answer in part d)? f. Speculate as to why any peak cluster with more than four peaks is listed simply as a"multiplet."arrow_forward
- Following are infrared spectra of nonane and 1-hexanol. Assign each compound its correct spectrum.arrow_forwarda)Can these two molecules be distinguished by IR spectroscopy? Give reasons for your answer and describe the main IR signals (with frequencies) you expect to see for both molecules. b)Can these two molecules be distinguished by mass spectrometry? Give reasons for your answer.arrow_forwardPrepare a table for the infrared spectroscopy results of key diagnostic peaks for both products and the starting alcohol. Products are 2-chloro-2-methylbuntane and 2-bromo-2-methylbutane. Alcohol is 2-methyl-2-buntanol. One column should include the wavenumber frequency and the other column should include the structure/vibration assignment (C(sp3)-H stretching, C-Br stretch, O-H stretch, etc.). Include any C-H stretching and bending frequencies, and likely C-Cl or C-Br frequencies.arrow_forward
- 1. True or False a. UV-Vis spectroscopy normally reports data in the from of bands rather than single peaks because of overlapping electronic transitions that are being recorded by the detector. (T/F) b. When using linear regression to translate absorption data using Beer's Law, the y-intercept (+b) of the linear equation represents the path length. (T/F) c. Phosphorescent materials give a glowing effect because the electrons remain at an excited state for much longer, which is the cause for the "glow". (T/F)arrow_forwardOn spectra 1 are the mass, IR and 13 C and 1 H NMRspectra of an organic compound.1) a) From these spectra, determine the structure of the molecule.Remember to ignore the triad in the 13 C NMR spectrum at 77 ppm thatcomes from the NMR solvent. b) Draw the structure of the molecule and label each hydrogen with a letter(A, B, C...). Then fill in the peak assignment table below. hydrogen chemical shift integration splitting pattern couples toarrow_forwardYour lab partner sends you a draft report summarising the luminescence measurements you both carried out on compounds A. The report describes the fluorescence of the molecule with a peak at 600 nm, absorption peak at 405 nm, and phosphorescence peak at 550 nm. What is your view on this information?arrow_forward
- 2. A. Match the following IR spectra with their respective molecules butane, 1-butene, and 1- butyne. Explain your answer. B. Assigned the major peaks in spectra (indicate the positions of vibrational modes belonging to the major functional groups in molecules). C. Calculate the number of vibrational modes in butane.arrow_forwardHow might you use IR spectroscopy to distinguish between the three isomers but-1-yne, buta-2-yne and buta-1,3-diene? Explain and justify your answer.arrow_forwardComplete the table with 3 Major IR peaks. IR Peak, cm-1 Bond Typearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY