
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14.1, Problem 2P
Interpretation Introduction
Interpretation: The given species is to be identified as conjugated.
Concept Introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
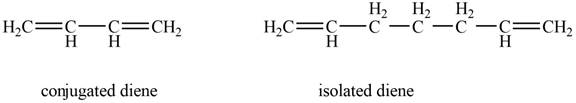
Figure 1
Allylic carbocation is also an example of conjugated system.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which carbocation would be lowest energy?
Options:
A
B
C
D
Which is an energy diagram for a concerted reaction (SN2 and E2)?
A
B
C
D
Out of SN1 and SN2, which reaction occurs with(a) Inversion of configuration(b) Racemisation
Chapter 14 Solutions
Organic Chemistry (6th Edition)
Ch. 14.1 - Prob. 2PCh. 14.2 - Problem 16.3 Draw a second resonance structure for...Ch. 14.2 - Prob. 4PCh. 14.2 - Problem 16.5 Farnesyl diphosphate is synthesized...Ch. 14.3 - Prob. 6PCh. 14.4 - Prob. 7PCh. 14.4 - Prob. 8PCh. 14.8 - Problem 16.12 Using hybridization, predict how the...Ch. 14.8 - Problem 16.13 Use resonance theory to explain why...Ch. 14.9 - Prob. 15P
Ch. 14.9 - Prob. 16PCh. 14.10 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 14.11 - Prob. 19PCh. 14.12 - Problem 16.19 Draw the product formed when each...Ch. 14 - Prob. 32PCh. 14 - 16.31 Which of the following systems are...Ch. 14 - 16.32 Draw all reasonable resonance structures for...Ch. 14 - Prob. 35PCh. 14 - 16.35 Explain why the cyclopentadienide anion A...Ch. 14 - Prob. 38PCh. 14 - 16.37 Draw the structure of each compound.
a. in...Ch. 14 - 16.41 Draw the products formed when each compound...Ch. 14 - Prob. 44PCh. 14 - 16.43 Treatment of alkenes A and B with gives the...Ch. 14 - 16.44 Draw a stepwise mechanism for the following...Ch. 14 - Prob. 47PCh. 14 - 16.57 A transannular Diels–Alder reaction is an...Ch. 14 - Draw a stepwise mechanism for the following...Ch. 14 - Prob. 62PCh. 14 - Prob. 63PCh. 14 - Prob. 64PCh. 14 - Prob. 65PCh. 14 - 16.65 The treatment of isoprene with one...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the neccessary reagents next to the arrowsarrow_forwardThe purine heterocycle occurs commonly in the structure of DNA.a.How is each N atom hybridized? b.In what type of orbital does each lone pair on a N atom reside? c.How many π electrons does purine contain? d.Why is purine aromatic?arrow_forward(a) Rank A, B, and C in order of increasing SN2 reactivity. (b) Rank A, B, and C in order of increasing SN1 reactivity.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning