
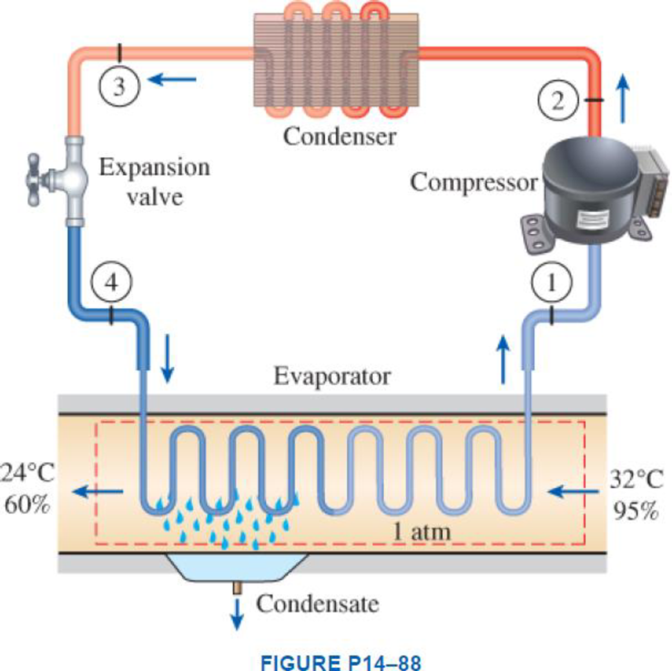
Atmospheric air at 1 atm, 32°C, and 95 percent relative humidity is cooled to 24°C and 60 percent relative humidity. A simple ideal vapor-compression refrigeration system using refrigerant-134a as the working fluid is used to provide the cooling required. It operates its evaporator at 4°C and its condenser at a saturation temperature of 39.4°C. The condenser rejects its heat to the atmospheric air. Calculate the exergy destruction, in kJ, in the total system per 1000 m3 of dry air processed.
The exergy destruction in the total system per
Answer to Problem 88P
The exergy destruction in the total system per
Explanation of Solution
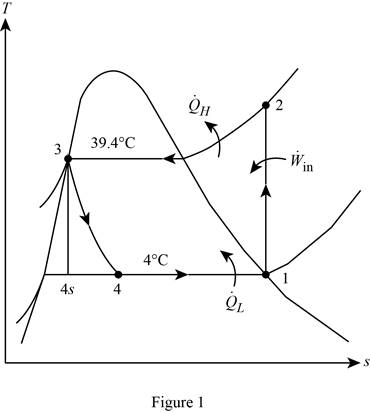
Show the T-s diagram for the simple ideal vapour-compression refrigeration system.
Express the mass of air.
Here, volume at state 1 is
Express the water mass balance and energy balance equations to the combined cooling and dehumidification section.
For water mass balance:
For energy balance:
Write the energy balance equation using steady-flow equation.
Here, the rate of total energy entering the system is
Substitute
Here, the specific enthalpy at the state 1 and 2 are
Write the formula for mass flow rate of refrigerant-134a.
Write the formula for amount of heat rejected from the condenser.
Calculate the exergy destruction in the components of the refrigeration cycle.
For the process 1-2,
Here, the process 1-2 is an isentropic.
For the process 2-3,
For the process 3-4,
Write the entropy change of water vapour in the air stream.
Write the entropy of water leaving the cooling section.
Determine the partial pressure of water vapour at state 1 for air steam.
Determine the partial pressure of dry air at state 1 for air steam.
Here, the pressure at the state 1 is
Determine the partial pressure of water vapour at state 2 for air steam.
Determine the partial pressure of dry air at state 2 for air steam.
Here, the pressure at the state 2 is
Write the formula for entropy change of dry air.
Write the formula for entropy change R-134a in the evaporator.
Write the formula for an entropy balance on the evaporator.
Write the formula for an exergy destruction in the evaporator.
Write the formula for the total exergy destruction.
Conclusion:
Refer Figure A-31, “psychometric chart at
Refer Table A-4, “saturated water-temperature table”, and write the specific enthalpy of condensate water at temperature of
Here, entropy of saturation liquid at temperature of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is temperature and specific enthalpy of condensate water at state 2 respectively.
Show the specific enthalpy of condensate water at corresponding to temperature as in Table (1).
|
Temperature |
Specific enthalpy of condensate water |
| 25 | 104.83 |
| 28 | |
| 30 | 125.74 |
Substitute
Substitute
Substitute
Substitute 1105 kg for
Substitute 1105 kg for
From the Table A-11 “Saturated Refrigerant-134a-Pressure Table”, obtain the value of the specific enthalpy and entropy at state 1 of
Refer Table A-13 “Saturated Refrigerant-134a-Pressure Table”, and write the specific enthalpy at state 2 in 1 MPa of pressure and entropy of
From the Table A-12 “Saturated Refrigerant-134a-Pressure Table”, obtain the value of the specific enthalpy and entropy at state 3 of
Here, the specific enthalpy at the state 4 and 3 are equal in the throttling.
Calculate the value of
From the Table A-11 “Saturated Refrigerant-134a-Pressure Table”, obtain the value of the specific enthalpy and entropy of saturated liquid and change upon vaporization at state 4 of
Substitute
Calculate the value of specific entropy of the state 4.
Substitute
Substitute
Substitute
Substitute
Substitute
Refer Table A-4 “Saturated water-temperature Table”, and write the specific entropy at state 1 at
Refer Table A-4 “Saturated water-temperature Table”, and write the specific entropy at state 1 at
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute 0 for
Thus, the exergy destruction in the total system per
Want to see more full solutions like this?
Chapter 14 Solutions
Thermodynamics: An Engineering Approach
- An air-conditioning system is to take in outdoor air at 10 C and 30 percent relative humidity at a steady rate of 45 m3/min and to condition it to 25 C and 60 percent relative humidity. The outdoor air is first heated to 22 C in the heating section and then humidified by the injection of hot steam in the humidifying section. Assuming the entire process takes place at a pressure of 100 kPa, determine a. the rate of heat supply in the heating section and b. the mass flow rate of the steam required in the humidifying section.arrow_forwardIf the partial pressure of water is 3Mpa and the corresponding saturated pressure is 3.5 Mpa, determine its relative humidity and water activity.arrow_forwardAn air-conditioning system is to take in air at 1 atm, 34 °C, and 70 % relative humidityand deliver it at 22 °C and 50 % relative humidity. The air flows first over the coolingcoils, where it is cooled and dehumidified, and then over the resistance heating wires,where it is heated to the desired temperature. Assuming that the condensate is removedfrom the cooling section at 10°C, determine a) the rate of heat supply in the heating section, and b) the mass flow rate of the steam required in the humidifying sectionarrow_forward
- Is it possible that during sensible cooling process the condition of air reaches a relative humidity of more than 100% or Is it possible that during sensible cooling process the condition of air reaches a relative humidity of 0%?arrow_forwardTwo humid airstreams are adiabatically mixed at 1 atm pressure to form a third stream. The first stream has a temperature of 100°F, a relative humidity of 90 percent, and a volume flow rate of 3 ft3/s, while the second stream has a temperature of 50°F, a relative humidity of 30 percent, and a volume flow rate of 1 ft3/s. Calculate the third stream’s temperature and relative humidity. Use data from the psychrometric chart. The third stream’s temperature is _________°F. The relative humidity is___________ %.arrow_forwardA dryer is to deliver 1,000 kg/hour of palay with a final moisture content of 10%. The initial moisture content in the feed is 15% at atmospheric condition with 32°C dry bulb and 21°C wet bulb. The dryer is maintained at 45°C while the relative humidity of the hot humid air from the dryer is 80%. If the steam pressure supplied to the heater is 2 MPaa, determine: a) amount of palay supplied to the dryer in kg/hr and the temperature of the hot humid air from the dryer in °C. b) air supplied to the dryer in m3/hr. c) heat capacity of the heater in kW; and d) steam supplied to the heater in kg/hr.arrow_forward
- An air-conditioning system operates at a total pressure of one atmosphere and consists of a heating section and an evaporative cooler. Air enters the heating section at 10 °C and 70 percent relative humidity at a rate of 30 m3/min, and it leaves the evaporative cooler at 20 °C and 60 percent relative humidity. Use this information to answer the following question. Choose the nearest value given in (a) – (e).The rate of water added to air in the evaporative cooler (kg/min) is (a) 0.12 (b) 0.67 (c) 13 (d) 37 (e) 67arrow_forwardAir enters the heating section of an air-conditioning at 1atm, 10C, and 70 percent relative humidity at a rate of 35m^3/min, and it leaves the humidifying section at 20C with a relative humidity of 90 percent. If the humidifier supplies wet steam at 100C, determine: The temperature and relative humidity of the air leaving the heating section The rate of heat transfer in the heating sectionarrow_forwardI need this with perfect answer 143103 An air-conditioning system operates at a total pressure of 1 atm and consists of a heating section and an evaporative cooler. Air enters the heating section at 15°C and 55 percent relative humidity at a rate of 30 m3 /min, and it leaves the evaporative cooler at 25°C and 45 percent relatively humidity. Determine (a) the temperature and relative humidity of the air when it leaves the heating section, (b) the rate of heat transfer in the heating section, and (c) the rate of water added to air in the evaporative cooler.arrow_forward
- Using the standard tables (Appendix C, Tables C-1 and C-2), determine the relative humidity and dew-point temperature if the dry-bulb thermometer reads 22°C and the wet-bulb thermometer reads 16°C. How would the relative humidity and dew point change if the wet-bulb reading were 19°C?arrow_forwardIt is desired to reduce the temperature of the ambient air entering an air handling unit at a volumetric flow rate of 550 m3/min, at a temperature of 35°C and a relative humidity of 0.75 to 20°C and its relative humidity to 0.45. For this purpose, the air is first cooled to a temperature below the dew point by passing through a cooling coil, and after some moisture is taken by condensation, its temperature is increased to 20˚C by passing over a heating coil. In this way, the temperature of the ambient air at the outlet of the air handling unit is brought to 20˚C and its relative humidity to 0.45. It is assumed that the pressure remains constant at P=100 kPa. Relationships will be used in the calculations. a) Calculate the amount of moisture condensed per unit time.b) Find the amount of heat drawn from the air by the cooling coil.c) Calculate the amount of heat given to the air by the heating coil.d) Show the phase changes on the psychrometric diagram.arrow_forwardUsing a psychrometric diagram, describe the heating and humidification process from the following data. Initially the air is heated at dry bulb temperature (DBT) 40 ° C and RH 55%. The air is heated to DBT 90 ° C. The heated air is then flowed through a humidifier to increase the RH to 25%. For this process, calculate a. change in absolute humidity of air from initial to final conditions = kg water / kg air b. change in enthalpy of air from initial to final conditions = kj / kg airarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





