
ORGANIC CHEMISTRY-EBOOK>I<
9th Edition
ISBN: 9781305084414
Author: McMurry
Publisher: INTER CENG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14.SE, Problem 61AP
Interpretation Introduction
Interpretation:
The concentration of the ergosterol solution whose absorbance A= 0.065 with a sample pathlength l = 1.00 cm is to be calculated if ergosterol has a UV absorption maxima of 282 nm and mole absorptivity ε = 11,900.
Concept introduction:
The molar absorptivity, ε, is given by 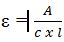 where A is absorbance, C is the concentration of the solution in moles/lit and l is the sample path length in cm.
where A is absorbance, C is the concentration of the solution in moles/lit and l is the sample path length in cm.
To calculate:
The concentration of the ergosterol solution whose absorbance A= 0.065 with a sample pathlength l = 1.00 cm, if ergosterol has a UV absorption maxima of 282 nm and mole absorptivity ε = 11,900.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Find the A and T% of a 6.5X104 mol L1 solution
of a drug (Molecular weight 200) whose
absorptivity is 0.026 L cm1 g'at 520 nm when
measured in a cell of 2.00 cm pathlength?
UV: max 235 (ε 12,000) and max 314 (ε 120)
EIMS: m/z 39 (31%), 41 (33%), 55 (37%), 69 (26%), 98 (100%), 99 (7%), 100 (0.2%)
IR:
Imm
IR spectrum in
CCl4 solvent
2950 cm-¹
2000
4) RC=N
5) 0-H
BAYEHUNIERI -
R
1630 cm-¹
1710 cm-¹
$10
4a) Which of the following heteroatoms that are definitely NOT present based on the EIMS data? (Circle
all that apply).
Br Cl FSI 0
4 continued) Please refer to data on preceding page.
4b) From the EIMS data what is the best estimate of the number of carbon atoms in this molecule?
4c) From the UV data, conjugated л-bonds are: definitely present definitely absent
4d) From the IR spectrum alone, which of the following functional groups can be definitely ruled OUT?
Circle those that are ruled out and briefly explain why.
1) C=0
2) RC=CR
3) C=C
cannot tell
Figure 1 shows a 1H NMR spectrum of a solution that was prepared by dissolving 1,3,5-trimethoxybenzene (10.22 mg) in CDCl3 (1.470 g).T
What is the concentration in mol dm–3 of CHCl3 present in the CDCl3? (Density of CDCl3 = 1.500 g mL–1.)
Chapter 14 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Ch. 14.1 - Prob. 1PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.2 - Prob. 3PCh. 14.2 - Give the structures of both 1, 2 and 1, 4 adducts...Ch. 14.3 - Prob. 5PCh. 14.3 - Prob. 6PCh. 14.5 - Predict the product of the following Diels–Alder...Ch. 14.5 - Prob. 8PCh. 14.5 - Which of the following dienes have an s-cis...Ch. 14.5 - Predict the product of the following Diels–Alder...
Ch. 14.6 - Prob. 11PCh. 14.6 - Prob. 12PCh. 14.7 - Prob. 13PCh. 14.7 - Prob. 14PCh. 14.8 - Which of the following compounds would you expect...Ch. 14.SE - Prob. 16VCCh. 14.SE - Show the product of the Diels–Alder reaction of...Ch. 14.SE - Prob. 18VCCh. 14.SE - Prob. 19VCCh. 14.SE - Prob. 20MPCh. 14.SE - Prob. 21MPCh. 14.SE - In light of your answer to Problem 14-21 propose...Ch. 14.SE - Luminol, which is used by forensic scientists to...Ch. 14.SE - Prob. 24MPCh. 14.SE - Give IUPAC names for the following compounds:Ch. 14.SE - Prob. 26APCh. 14.SE - Prob. 27APCh. 14.SE - Electrophilic addition of Br2 to isoprene...Ch. 14.SE - Prob. 29APCh. 14.SE - Prob. 30APCh. 14.SE - Predict the products of the following...Ch. 14.SE - 2,3-Di-tert-butyl-1,3-butadiene does not undergo...Ch. 14.SE - Prob. 33APCh. 14.SE - Prob. 34APCh. 14.SE - Prob. 35APCh. 14.SE - Prob. 36APCh. 14.SE - Rank the following dienophiles in order of their...Ch. 14.SE - Prob. 38APCh. 14.SE - Prob. 39APCh. 14.SE - Prob. 40APCh. 14.SE - Although the Diels–Alder reaction generally...Ch. 14.SE - Prob. 42APCh. 14.SE - Tires whose sidewalls are made of natural rubber...Ch. 14.SE - Prob. 44APCh. 14.SE - Prob. 45APCh. 14.SE - Prob. 46APCh. 14.SE - Would you expect allene, H2C = C = CH2, to show a...Ch. 14.SE - The following ultraviolet absorption maxima have...Ch. 14.SE - Prob. 49APCh. 14.SE - -Ocimene is a pleasant-smelling hydrocarbon found...Ch. 14.SE - Draw the resonance forms that result when the...Ch. 14.SE - Prob. 52APCh. 14.SE - Treatment of 3,4-dibromohexane with strong base...Ch. 14.SE - Prob. 54APCh. 14.SE - Prob. 55APCh. 14.SE - Prob. 56APCh. 14.SE - Prob. 57APCh. 14.SE - Prob. 58APCh. 14.SE - Hydrocarbon A, C10H14, has a UV absorption at...Ch. 14.SE - Prob. 60APCh. 14.SE - Prob. 61APCh. 14.SE - Prob. 62APCh. 14.SE - Prob. 63APCh. 14.SE - Prob. 64APCh. 14.SE - The double bond of an enamine (alkene + amine) is...Ch. 14.SE - Prob. 66AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nitriles, R–=C≡N, undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the compound produced by hydrolysis of propanenitrile, CH3CH2C≡N, if it has IR absorptions from 2500–3100 cm-1 and at 1710 cm-1, and has M+=74?arrow_forwardHow could 1H NMR spectroscopy be used to distinguish among isomersA, B, and C?arrow_forward6. (Chapter 15 - 47b) An aromatic compound, C9H12 has the following H NMR spectrum and a peak at 750 cmt in its IR spectrum. Answer the following questions. Chem. shift Rel. area 1.19 2.31 2.64 7.13 1.50 1.50 1.00 2.00 TMS 10 3 O ppm Chemical shift (6) 4(a). The IR peak at 750 cm in its IR spectrum indicate that the compound is. disubstituted = 4(b) The name of the compound is = Intensity-arrow_forward
- What functional group can you get from its IR spectrum? Support your answer by identifying prominent peaks (wavenumbers cm^-1) and assigning the functional group.arrow_forwardA student performed a series of reactions and obtained the spectral data provided. The reaction sequence is shown below.. What are the structures for A, B, C, and D? Compound B NMR (relative integrated areas below peaks) CH;CH,O°N 1. BHTHF N-bromosuccnimide A CH;CH,OH B 2. H,O,/OH- D hv reflux Compound A NMR (relative integrated areas below peaks) PPM 5 Compound B IR INFRARED SPECTRUM PPM 5 1 6 0.8 Compound A IR INFRARED SPECTRUM 0.6 0.8 0.4 0.4 0.2- 3000 2000 Wavenumber (cm-1) 1000 The MS of compound B shows an M+ peak at 198 and an M+2 peak at 200 with a relative abundance of 1:0.97 2000 Wavenumber (cm-1) 3000 1000 Transmitance Relative Transmittancearrow_forwardCHM 251 psession 1 Feb 1 For each molecular ion listed below, indicate what element(s) besides C and H must be in the compound: 107 162 and 164; 3:1 intensity ratio 162 and 164; 1:1 intensity ratio 220, 222, and 224; 1:2:1 intensity ratio 187 and 189; 3:1 intensity ratioarrow_forward
- How could 1H NMR spectroscopy be used to distinguish among isomers A, B, and C?arrow_forwardThe triple amine in an acidic medium does not give a transition n→o * Explain why?arrow_forwardA compound A of mass 114 has the following elemental analysis: C = 63.14%, H=8.83%. The IR, 1H NMR and 13C NMR spectra are given below. 1) Assign and interpret all the data from the 1H NMR and 13C NMR spectra. Propose a formula for A. 7.0 175 с 3000 1,3; 13 I=1 6.0 150 I=1 с 5.0 CH₂ 125 2000 Explain why the answer is this one : CH,—CH,—0 4,2; 60 mavermy I=2 cm-1 4.0 100 1500 3.0 75 135 167 | CH₂ 6 125 CH 3 1,95 ; 18 I=3 1000 2.0 50 H 5,5 I=3 1.0 CH₂ CH₂ 25 500 Ppm ppmarrow_forward
- This question has multiple parts. Work all the parts to get the most points. a Give the structure for the compound with the empirical formula C3H3O2 and the following spectral data: 1Η ΝMR Singlet at 3.7 ppm (3H); multiplet at 7.2 ppm (5H) IR 2850 cm1 (sharp), 1720 cm-1, 1600 cm-1, 1503 cm! 100- 105 80 77 60- M: 136 40- ae 20- 40 80 120 160 miz % Relative abundancearrow_forwardThe leaves of the Brazilian Tree Senna multijunga contain a number of pryidine alkaloids that inhibit acetylcholinterinase. Two recentyl isolated isomeric compounds have the strcture have the strcture shown below. (NOTE: M=293) Use the mass spectral data provided to determine the precise location of the hydroxyl group in each isomer. Isomer A: EI-MS, m/z(rel. int): 222(20), 150(10), 136(25), 123(100) Isomer B:EI-MS, m/z(re;. int): 236(20), 150(10), 136(25), 123(100)arrow_forwardIdentify the reagent used in the following transformation: OH P Q HO OH HO (A) P = LIAIH, Q = KMNO4 Q = MnO, / CH,Cl, Q= Ag,CO, on celite, PhH %3D (В) P= NABH, /MeOH %3D (C) P=CeCl,.7H,O (D) P= NABH, /CeCl,.7H,0 , / CeCl,.7H,O Q = MnO, / CH,Cl,arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning