Concept explainers
15-21 Answer true or false.
- For a molecule with two stereocenters, 22 = 4 stereoisomers are possible.
Diastereomers are stereoisomers that are not mirror images.
(a)
Interpretation:
To analyze whether the given statement- For a molecule with two stereocenters, 22 =4 stereoisomers are possible, is true or false.
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
Answer to Problem 15.21P
For a molecule with two stereocenters, 22 =4 stereoisomers are possible. Thus, given statement is true.
Explanation of Solution
According to van't Hoff rule, if a molecule contains n number of chiral carbons, provided it does not contain any elements of symmetry, and then the number of stereoisomers is given by- 2n.
Therefore for a molecule with 2 stereocenters, the possible isomer is given as-
22 =4.
Therefore, 4 stereoisomers are possible.
(b)
Interpretation:
To analyze whether the given statement- For a molecule with three stereocenters, 32 =9 stereoisomers are possible, is true or false.
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
A molecule is said to be chiral if it cannot be superimposed on its mirror image and if it does not possess an alternate axis of symmetry.
A carbon atom bonded in a tetrahedral structure to four different substituents in a molecule, it is called as a chiral centre or stereocentre.
If an organic molecule has more than one chiral carbon or chiral centre then the molecule may be achiral or chiral and it is depend upon whether the molecule has element of symmetry or not.
Answer to Problem 15.21P
For a molecule with three stereocenters, 32 =9 stereoisomers are possible. Thus, given statement is true.
Explanation of Solution
According to van't Hoff rule, if a molecule contains n number of chiral carbons, provided it does not contain any elements of symmetry, and then the number of stereoisomers is given by- 2n.
Therefore for a molecule with 3 stereocenters, the possible isomer is given as-
32 =9.
Therefore, 9 stereoisomers are possible.
(c)
Interpretation:
To analyze whether the given statement- Enantiomers, like gloves, occur in pairs, is true or false.
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. The phenomenon of enantiomerism is called as optical isomerism. If the configuration at each stereocenter is changed, the enantiomer is converted into another enantiomer.
Answer to Problem 15.21P
Enantiomers, like gloves, occur in pairs.
Explanation of Solution
Isomers which are non-superimposable mirror images of each other are called Enantiomers.
Compounds with a chiral centre exist in enantiomeric forms. The phenomenon of enantiomerism is called as optical isomerism. If the configuration at each stereocenter is changed, the enantiomer is converted into another enantiomer.
Hence, Enantiomers, like gloves, occur in pairs.
(d)
Interpretation:
To analyze whether the given statement- 2-Pentanol and 3-Pentanol are both chiral and show enantiomerism, is true or false.
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. The phenomenon of enantiomerism is called as optical isomerism. If the configuration at each stereocenter is changed, the enantiomer is converted into another enantiomer.
Answer to Problem 15.21P
Only 2-Pentanol is chiral and show enantiomerism.
Explanation of Solution
Isomers which are non-superimposable mirror images of each other are called Enantiomers.
In 2-Pentanol, the carbon-2 is attached to four different groups namely- a methyl group, a propyl group, hydroxyl group and hydrogen.
Therefore it has a stereocenter, it forms a mirror image that is non-superimposable, and therefore they show isomerism.
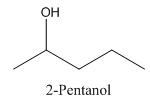 has a mirror image as
has a mirror image as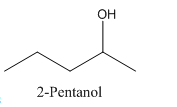
In 3-Pentanol, the carbon-2 is attached to four groups namely- two ethyl group, hydroxyl group and hydrogen.
Therefore it does not have a stereocenter, it forms a mirror image that is superimposable, and therefore they do not show isomerism.
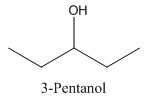 has a mirror image as
has a mirror image as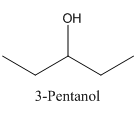
(e)
Interpretation:
To analyze whether the given statement- 1-Methylcyclohexanol is achiral and does not show enantiomerism, is true or false.
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. The phenomenon of enantiomerism is called as optical isomerism. If the configuration at each stereocenter is changed, the enantiomer is converted into another enantiomer.
Answer to Problem 15.21P
1-Methylcyclohexanol is achiral and does not show enantiomerism.
Explanation of Solution
1- Methylcyclohexanol has a plane of symmetry due to which it is not optically active.
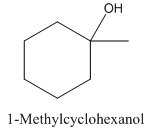
1- Methylcyclohexanol is achiral and doesn’t show enantiomerism.
(f)
Interpretation:
To analyze whether the given statement- Diastereomers are stereoisomers that are not mirror images, is true or false.
Concept Introduction:
Stereoisomerism deals with the study of the spatial arrangements of the atoms in space. It does not mean to alter the connectivity of the atoms by mean of forming or breaking the bonds.
Compounds with a chiral centre exist in enantiomeric forms. The phenomenon of enantiomerism is called as optical isomerism. If the configuration at each stereocenter is changed, the enantiomer is converted into another enantiomer.
Answer to Problem 15.21P
Diastereomers are Stereoisomers that are not mirror images.
Explanation of Solution
Stereoisomers which don't have any mirror image relationship are called Diastereomers.
Diastereomers are non-enantiomeric stereoisomers which have two or more stereocenter and differ only in configuration of at least one of them.
Example-
In tartaric acid,
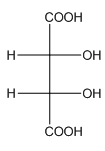 and
and 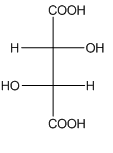 are diastereomers.
are diastereomers.
Want to see more full solutions like this?
Chapter 15 Solutions
Introduction to General, Organic and Biochemistry - Access
- 15-44 Consider the structure of the immunosuppressant FK-506, a molecule shown to disrupt calcineurin- mediated signal transduction in T-lymphocytes. What is the molecular formula of this immunosuppressant? How manj' stereocenters are present in FK-506? Determine the maximum number of stereoisomers possible. Identify and label the various functional groups present. Consider the two stereocenters in this structure labeled with asterisks (*). Determine the absolute configuration of each stereocenter. FK-506 has been shown to exhibit moderate solubility in various organic solvents. Is this immunosuppressant expected to be soluble in ethanol (CH3CH2OH)? Consider the carbon atom labeled “1." Describe the geometry and approximate bond angles about this carbon atom. Draw the alternative chair conformations of the cyclohexane ring at the lower right of FK-506 and label the more stable conformation. Are there any aromatic components present in FK-506? Patients taking FK-506 have reported several side effects from this medication, including headaches, nausea or diarrhea, and slight shaking. Would you expect the enantiomer of this drug to result in the same side effects?arrow_forward15-37 Consider a cyclohexane ring substituted with one hydroxyl group and one methyl group. Draw a structural formula for a compound of this composition that: Does not show cis-trans isomerism and has no stereocenters. Shows cis-trans isomerism but has no stereocenters. Shows cis-trans isomerism and has two stereocenters.arrow_forward15-43 Triamcinolone acetonide, the active ingredient in Azmacort Inhalation Aerosol, is a steroid used to treat bronchial asthma. Triamcinolone acetonide Label the eight stereocenters in this molecule. How many stereoisomers are possible for it? (Of these, the stereoisomer with the configura tion shown here is the active ingredient in Azmacort.)arrow_forward
- 15-16 Which of the following compounds contain stereocenters? (a) 2-Chloropentane(b) 3-Chloropentane (c) 3-Chloro-l-butene(d) 1,2-Dichloropropanearrow_forward15-35 Following are structural formulas for three of the drugs most widely prescribed to treat depression. Label all stereocenters in each and state the number of stereoisomers possible for each. Fluoxetine (Prozac!arrow_forward15-26 For centuries, Chinese herbal medicine has used extracts oi Ephedra sinica to treat asthma. The asthma-relieving component of this plant is ephedrine, a very potent dilator of the air passages of the lungs. The naturally occurring stereoisomer is levorotatory and has the following structure. HO ¥ Nhch3 Ephedrine |n|„ = —41° Mark each stereocenter in epinephrine with an asterisk. How many stereoisomers are possible for this compound?arrow_forward
- 15-17 Which of the following compounds contain stereocenters? Cyclopentanol l-Chloro-2-propanol 2-Methylcyclopentanol 1-Phenyl-l-propanolarrow_forward15-7 Answer true or false. The cis and trans stereoisomers of 2-butene are achiral. The carbonyl carbon of an aldehyde, ketone, carboxylic acid, or ester cannot be a stereocenter. Stereoisomers have the same connectivity of their atoms. Constitutional isomers have the same connectivity of their atoms. An unmarked cube is achiral. A human foot is chiral. Every object in nature has a mirror image. The most common cause of chirality in organic molecules is the presence of a tetrahedral carbon atom with four different groups bonded to it. If a molecule is not superposable on its mirror image, the molecule is chiral.arrow_forward13-29 Show that if you add Steps 2a and 2b of the radical- chain mechanism for the autoxidation of a fatty acid hydrocarbon chain, you arrive at the following net equation: H I —CH2CH=CH—CH— + 0—0 • » Section of a fatty acid Oxygen hydrocarbon chain O—O—H I —CH2CH=CH—CH— A hydroperoxidearrow_forward
- 15-8 What does the term “chiral” mean? Give an example of a chiral molecule.arrow_forward17-54 Following is the structure of immunosuppressant FK-506, a molecule shown to disrupt calcineurin-mediated signal transduction in T-lymphocytes. (a) There are three carbon—carbon double bonds present in this molecule. Which of the three has the potential for cis/trans isomerism? Assign a cis or trans con?guration to each carbon-carbon double bond that has this possibility. (b) How many stereocenters are present in this molecule? How many stereoisomers are possible for it? (c) Are there any aromatic components in this molecule? (d) Consider the two carbon atoms marked with asterisks. Assign an R or S con?guration of each stereocenter. (e) Because of the presence of a 21-member ring, this molecule is described as a macrocycle. This ring is fashioned by three types of bonds, several carbon-carbon bonds, one ester, one hemiacetal, and one amide. Locate the ester and the hemiacetal. (f) Draw the structural formula of the long chain compound that would result if the hemiacetal were to be cleaved to an alcohol and a carbonyl group.arrow_forward17-74 Glucose, C6H12O6, contains an aldehyde group but exists predominantly in the form of the cyclic hemiacetal shown here. We will discuss this cyclic form of glucose in Chapter 20. A cyclic hemiacetal is formed when the —OH group of one carbon bonds to the carbonyl group of another carbon. (a) Which carbon in glucose provides the —OH group and which provides the —CHO group? (b) Draw the alternative chair confirmations of D-glucose and state which of the two is the more stable.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
