
(a)
Interpretation: The products for radical chlorination and bromination of given compound are to be drawn. The compounds which form single constitutional isomer for both reactions are to be predicted. The true structure of a reactant for both reactions to form a single product is to be identified.
Concept introduction: Chlorination and bromination are radical substitution reaction. In the reaction of chlorination,
Answer to Problem 15.41P
The products of radical chlorination and bromination of given compound are,
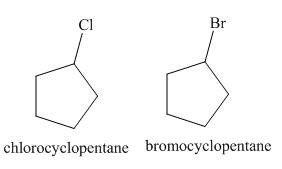
A single constitutional isomer is formed for both reactions.
Explanation of Solution
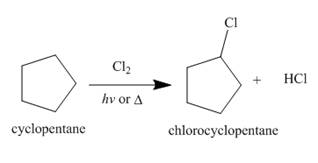
Figure 1
Alkanes undergo bromination through free radical mechanism when they are treated with
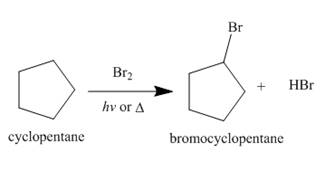
Figure 2
A single constitutional isomer is formed for both reaction because all hydrogen atoms of given compound are present in a same chemical environment. Therefore, to form a single product, the chemical environment of hydrogen atoms should be same.
The products of radical chlorination and bromination of given compound are shown in Figure 1 and Figure 2, respectively. A single constitutional isomer is formed for both reactions.
(b)
Interpretation: The products for radical chlorination and bromination of given compound are to be drawn. The compounds which form single constitutional isomer for both reactions are to be predicted. The true structure of a reactant for both reactions to form a single product is to be identified.
Concept introduction: Chlorination and bromination are radical substitution reaction. In the reaction of chlorination,
Answer to Problem 15.41P
The products of radical chlorination and bromination of given compound are,
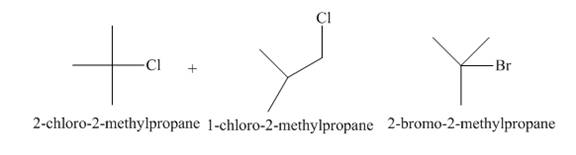
A single constitutional isomer is not formed for both reactions.
Explanation of Solution
Alkanes undergo chlorination when they are treated with
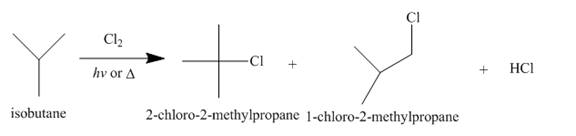
Figure 3
Alkanes undergo bromination by free radical mechanism when they are treated with
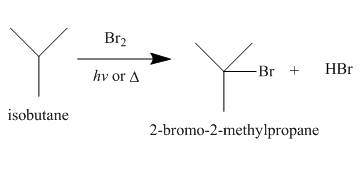
Figure 4
A single constitutional isomer is formed for bromination reaction but not for chlorination because bromine is more selective than chlorine.
The products of radical chlorination and bromination of given compound are shown in Figure 3 and Figure 4, respectively. A single constitutional isomer is not formed for both reactions.
(c)
Interpretation: The products for radical chlorination and bromination of given compound are to be drawn. The compounds which form single constitutional isomer for both reactions are to be predicted. The true structure of a reactant for both reactions to form a single product is to be identified.
Concept introduction: Chlorination and bromination are radical substitution reaction. In the reaction of chlorination,
Answer to Problem 15.41P
The products of radical chlorination and bromination of given compound are,
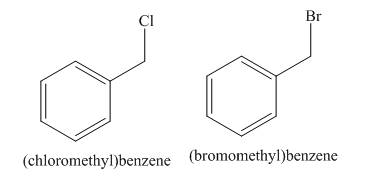
A single constitutional isomer is formed for both reactions.
Explanation of Solution
Alkanes undergo chlorination when they are treated with
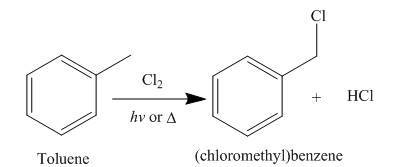
Figure 5
Alkanes undergo bromination when they are treated with
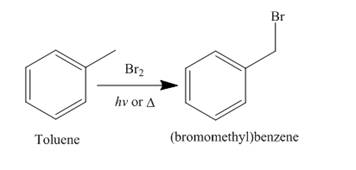
Figure 6
A single constitutional isomer is formed for both reaction because all hydrogen atoms of given compound are present in same chemical environment. Therefore, to form a single product, the chemical environment of hydrogen atoms should be same.
The products of radical chlorination and bromination of given compound are shown in Figure 5 and Figure 6, respectively. A single constitutional isomer is formed for both reactions.
(d)
Interpretation: The products for radical chlorination and bromination of given compound are to be drawn. The compounds which form single constitutional isomer for both reactions are to be predicted. The true structure of a reactant for both reactions to form a single product is to be identified.
Concept introduction: Chlorination and bromination are radical substitution reaction. In the reaction of chlorination,
Answer to Problem 15.41P
The products of radical chlorination and bromination of given compound are,
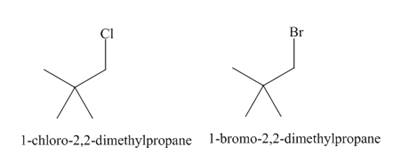
A single constitutional isomer is formed for both reactions.
Explanation of Solution
Alkanes undergo chlorination when they are treated with
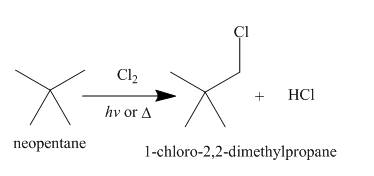
Figure 7
Alkanes undergo bromination when they are treated with
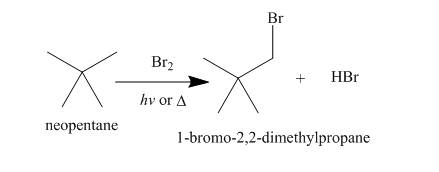
Figure 8
A single constitutional isomer is formed for both reaction because all hydrogen atoms of given compound are present in same chemical environment. Therefore, to form a single product, the chemical environment of hydrogen atoms should be same.
The products of radical chlorination and bromination of given compound are shown in Figure 7 and Figure 8, respectively. A single constitutional isomer is formed for both reactions.
(e)
Interpretation: The products for radical chlorination and bromination of given compound are to be drawn. The compounds which form single constitutional isomer for both reactions are to be predicted. The true structure of a reactant for both reactions to form a single product is to be identified.
Concept introduction: Chlorination and bromination are radical substitution reaction. In the reaction of chlorination,
Answer to Problem 15.41P
The products of radical chlorination and bromination of given compound are,
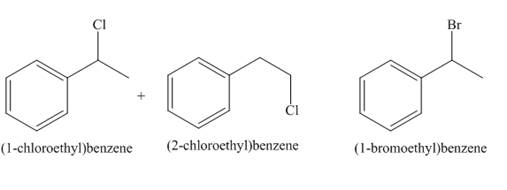
A single constitutional isomer is not formed for both reactions.
Explanation of Solution
Alkanes undergo chlorination when they are treated with
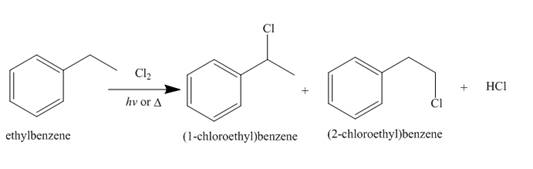
Figure 9
Alkanes undergo bromination by free radical mechanism when they are treated with
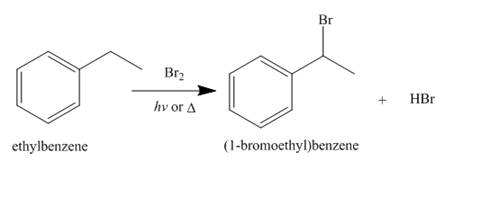
Figure 10
A single constitutional isomer is formed for bromination reaction but not for chlorination because bromine is more selective than chlorine.
The products of radical chlorination and bromination of given compound are shown in Figure 9 and Figure 10, respectively. A single constitutional isomer is not formed for both reactions.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry - With Access (Looseleaf) (Custom)
- What are the suitable reagent(s) needed for the transformations in a) and b)?arrow_forwardDevise a synthesis of each compound from cyclopentane and any other required organic or inorganic reagents.arrow_forwardDevise a synthesis of each compound from acetylene and any other required reagents.arrow_forward
- Draw a stepwise mechanism for the following reaction that forms ether D. D can be converted to the antidepressant fluoxetine (trade name Prozac) in a single step.arrow_forwardDevise a stepwise synthesis of attached compound from dicyclopentadieneusing a Diels–Alder reaction as one step. You may also use organiccompounds having ≤ 4 C's, and any required organic or inorganicreagents.arrow_forwardDraw the organic product of each reaction (a, b and c)arrow_forward
- Devise a synthesis of each compound from cyclohexene and any required organic or inorganic reagents.arrow_forwardDraw a stepwise mechanism for the following Robinson annulation. This reaction was a key step in a synthesis of the steroid cortisone by R. B. Woodward and co-workers at Harvard University in 1951.arrow_forwardDevise a synthesis of each compound from the indicated starting material:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





