
Concept explainers
Draw the organic products formed in each reaction.
a. ![]() d.
d. ![]() g.
g. 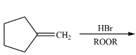
b.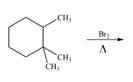 e.
e. 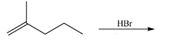 h.
h.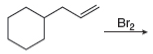
c. ![]() f.
f. 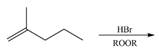 i.
i.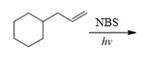
(a)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
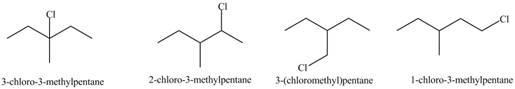
Explanation of Solution
Alkanes undergo chlorination when they are treated with
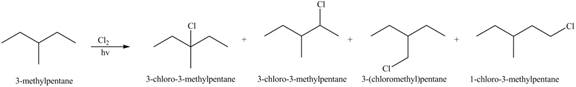
Figure 1
The organic products formed by the given reaction are shown in Figure 1.
(b)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
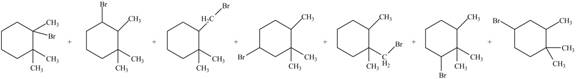
Explanation of Solution
Alkanes undergo bromination by free radical mechanism when they are treated with
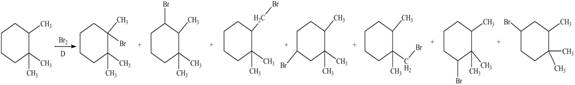
Figure 2
The organic products formed by the given reaction are shown in Figure 2.
(c)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
Explanation of Solution
Alkanes undergo bromination by free radical mechanism when they are treated with
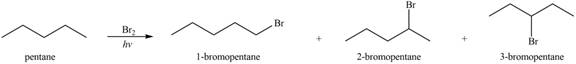
Figure 3
The organic products formed by the given reaction are shown in Figure 3.
(d)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
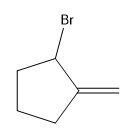
Explanation of Solution
Alkenes undergo bromination by free radical mechanism when they are treated with NBS in the presence of light. Bromination takes place at allylic carbon atom. One product is formed by the given bromination reaction as shown in Figure 3.
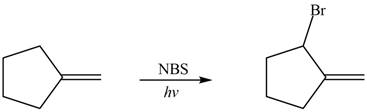
Figure 4
The organic products formed by the given reaction are shown in Figure 4.
(e)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
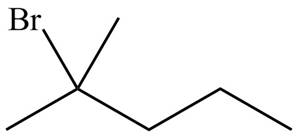
Figure 5
Explanation of Solution
Alkenes undergo addition reaction when they are treated with
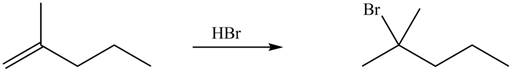
Figure 5
The organic products formed by the given reaction are shown in Figure 5.
(f)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
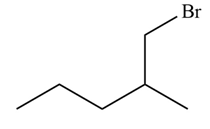
Explanation of Solution
Alkenes undergo addition reaction when they are treated with
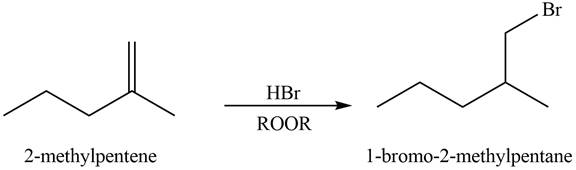
Figure 6
The organic products formed by the given reaction are shown in Figure 6.
(g)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
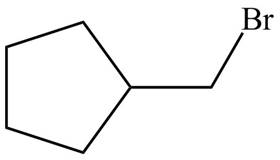
Explanation of Solution
Alkenes undergo addition reaction when they are treated with
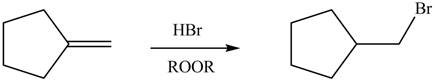
Figure 7
The organic products formed by the given reaction are shown in Figure 7.
(h)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
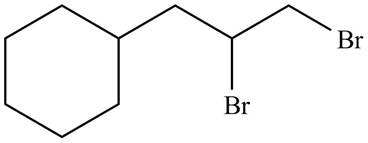
Explanation of Solution
Alkenes undergo addition reaction when they are treated with
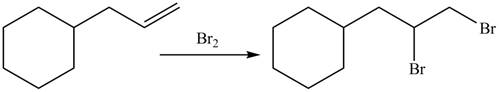
Figure 8
The organic products formed by the given reaction are shown in Figure 8.
(i)
Interpretation: The organic products formed by the given reaction are to be drawn.
Concept introduction: Alkenes and alkynes show addition reaction due to presence of one and two pi-bonds, respectively. Alkanes show substitution reaction due to absence of pi-bond.
Answer to Problem 15.48P
The organic products formed by the given reaction are shown below.
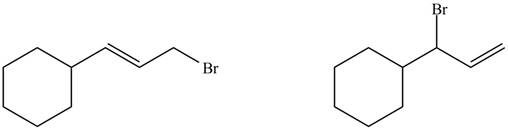
Explanation of Solution
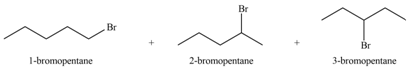
Alkenes undergo bromination by free radical mechanism when they are treated with NBS in the presence of light. Bromination takes place at allylic carbon atom. Two different products are formed by the given bromination reaction as shown in Figure 9.
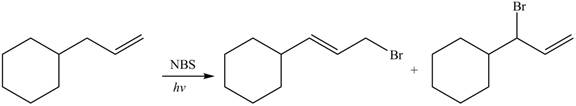
Figure 9
The organic products formed by the given reaction are shown in Figure 9.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry - With Access (Looseleaf) (Custom)
- Draw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form asubstituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that canbe used as a synthetic detergent to clean away dirt.arrow_forwardDraw a stepwise mechanism for the following reaction that forms ether D. D can be converted to the antidepressant fluoxetine (trade name Prozac) in a single step.arrow_forwarddraw compound a,b, and carrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





