
Concept explainers
(a)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, the frequency range for the given type of bond is identified by the
Answer to Problem 15.41P
An OH stretch with stretching frequency
Explanation of Solution
The given reaction is
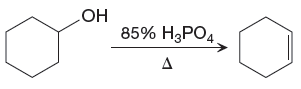
In the above reaction, the OH group, that is the alcohol functional group, is present in the reactant, and C=C bond of
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(b)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
A very broad OH stretch having stretching frequency
Explanation of Solution
The given reaction is
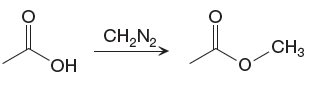
The
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(c)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
Two
Explanation of Solution
The given reaction is,
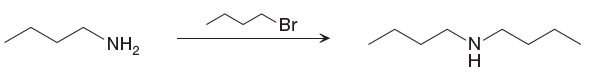
Two
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(d)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
The single N-H band having stretching frequency
Explanation of Solution
The given reaction is
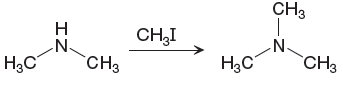
The single N-H band having stretching frequency
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(e)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
The
Explanation of Solution
The given reaction is
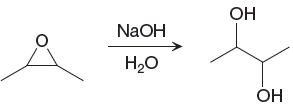
The epoxide having ether functional group would disappear from the reactant, and an OH band having stretching frequency
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(f)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
The
Explanation of Solution
The given reaction is
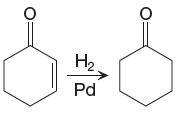
In the above reaction, the
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(g)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
The
Explanation of Solution
The given reaction is
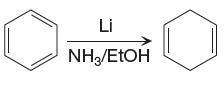
The
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(h)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
The strong
Explanation of Solution
The given reaction is
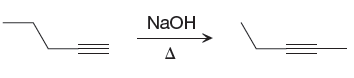
The strong
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
(i)
Interpretation:
The differences in the IR spectra of the reactant and product that would enable you to tell that the given reaction has taken place are to be determined.
Concept introduction:
The frequencies of the stretching vibrations are estimated from the type of bond and the frequency range given for that bond. Also, frequency range for the given type of bond is identified by the functional group to which that bond resembles in the molecule.
Answer to Problem 15.41P
The
Explanation of Solution
The given reaction is
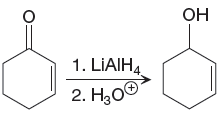
The reactant is conjugated ketone in the above reaction. The alcohol and alkene functional groups are present in the product. The
The differences in the IR spectra that would enable to tell that the given reaction has taken place are determined on the basis of bonds and the functional groups present in the reactant and the product.
Want to see more full solutions like this?
Chapter 15 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Reduction of cyclohex-2-enone can yield cyclohexanone, cyclohex-2enol, or cyclohexanol, depending on the reagent and reaction conditions. How could you use IR spectroscopy to distinguish the three possible products?arrow_forwardidentify the peaks in this ir spectraarrow_forwardHow would the attached pair of compounds differ in their IR spectra?arrow_forward
- Which of these compounds matches to the IR spectrum?arrow_forwarda) What differences in the IR spectra of the reactant and the product would enable you to tell that each of the following reaction took place? b) How would you use Grignard reagent on an aldehyde or ketone to synthesis the following compounds? ( attachements are folowed respectively)arrow_forwardHow can you distinguish between aldehydes and ketones based on Infra-red spectroscopy?arrow_forward
- Which of the following compounds matches the given IR spectrum?arrow_forwardDetermine which of the following belongs to this ir spectra then complete the nmr analysis methyl butanoate benzaldehyde 1-chlorobutane 1-chloro-2-methylpropane butan-2-one propan-2-ol propanalarrow_forwardThe alkene C8H8 was made when reacting an unknown carbonyl compound with methylene phosphonium ylide. The IR and mass spec for the unknown carbonyl compound and C8H8 are attached. Which spectrum is associated with which compound , and how do important peaks indicate this?arrow_forward
- A. Draw the structure of the synthesized product (do not include side or by products) from the experiments performed. How can IR spectroscopy be used to monitor the progress of each of the following reactions (if reaction went to completion and/or check the purity of the final product)? What absorptions would be observed in the IR spectrum? And if IR spectroscopy could not have been used, briefly explain why.arrow_forwardThis is the spectrum of the product of the synthesis of a Grignard reagent with an aldehyde. What peaks better demonstrate the presence of the predicted product?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
