
Concept explainers
Predict the major products of the following reactions:
(a)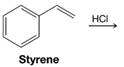
(b) 2-Bromo-1-phenylpropane
(c)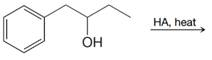
(d) Product of (c) + HBr
(e) Product of (c) +
(f) Product of (c) +
(g) Product of (f)
(h)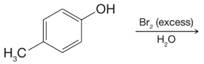
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Fundamentals of Heat and Mass Transfer
Chemistry: Matter and Change
Chemistry: The Central Science (14th Edition)
Principles of Chemistry: A Molecular Approach (3rd Edition)
General, Organic, and Biological Chemistry (3rd Edition)
General Chemistry: Atoms First
- Amines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardA step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardWhen 2,2-dibromo-1-phenylpropane is heated overnight with sodium amide at 150 °C, the major product (after addition of water) is a different foul-smelling compound of formula C9H8. Propose a structure for this product, and give a mechanism to account for its formation.arrow_forward
- The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nm and a weaker absorption at 258 nm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer with an intense absorption at 250 nm and a weaker absorption at 290 nm. Suggest a structure for the isomeric product and propose a mechanism for its formation.arrow_forwardHydroboration of 2-methyl-2-pentene at 25°C, followed by oxidation with alkaline H2O2, yields 2-methyl-3-pentanol, but hydroboration at 160°C followed by oxidation yields 4- methyl-l-pentanol. Suggest a mechanism.arrow_forwardHydroboration of 2-methyl-2-pentene at 25°C, followed by oxidation with alkaline H2o2, yields 2-methyl-3-pentanol, but hydroboration at 160°C followed by oxidation yields 4-methyl-1-pentanol. Suggest a mechanism.arrow_forward
- Predict the major products of the following reactions. n-butyl tosylate + sodium acetylide, H¬C‚C:- +Naarrow_forwardTreatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forwardShow the products you would obtain by acid-catalyzed reaction of cyclohexanone with ethylamine, CH3CH2NH2, and with diethylamine, (CH3CH2) 2NH.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

