
Concept explainers
(a)
Interpretation:
After volume of
Concept Introduction:
The relationship between
The
The
The relationship between
The concentration of
The concentration of
(a)
Explanation of Solution
Given,
The titration of
Volume of the solution is given below,
Mole of hydroxide ion is calculated as follows,
The
The
The
(b)
Interpretation:
After volume of
Concept Introduction:
Refer to part (a)
(b)
Explanation of Solution
Given,
The titration of
Volume of the acid solution is given below,
Mole of hydronium ion is calculated as follows,
The concentration of
The
The
The
(c)
Interpretation:
After volume of
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
Given,
The titration of
Volume of the acid solution is given below,
Mole of hydronium ion is calculated as follows,
The concentration of
The
The
The
(d)
Interpretation:
After volume of
Concept introduction:
Refer to part (a)
(d)
Explanation of Solution
Given,
The titration of
Volume of the acid solution is given below,
Mole of hydronium ion is calculated as follows,
The total moles of hydronium ion
(e)
Interpretation:
After volume of
Concept introduction:
Refer to part (a)
(e)
Explanation of Solution
Given,
The titration of
Volume of the acid solution is given below,
Mole of hydronium ion is calculated as follows,
The concentration of
The
The
The
(f)
Interpretation:
After volume of
Concept Introduction:
Refer to part (a)
(f)
Explanation of Solution
Given,
The titration of
Volume of the acid solution is given below,
Mole of hydronium ion is calculated as follows,
The concentration of
The
The
The
(g)
Interpretation:
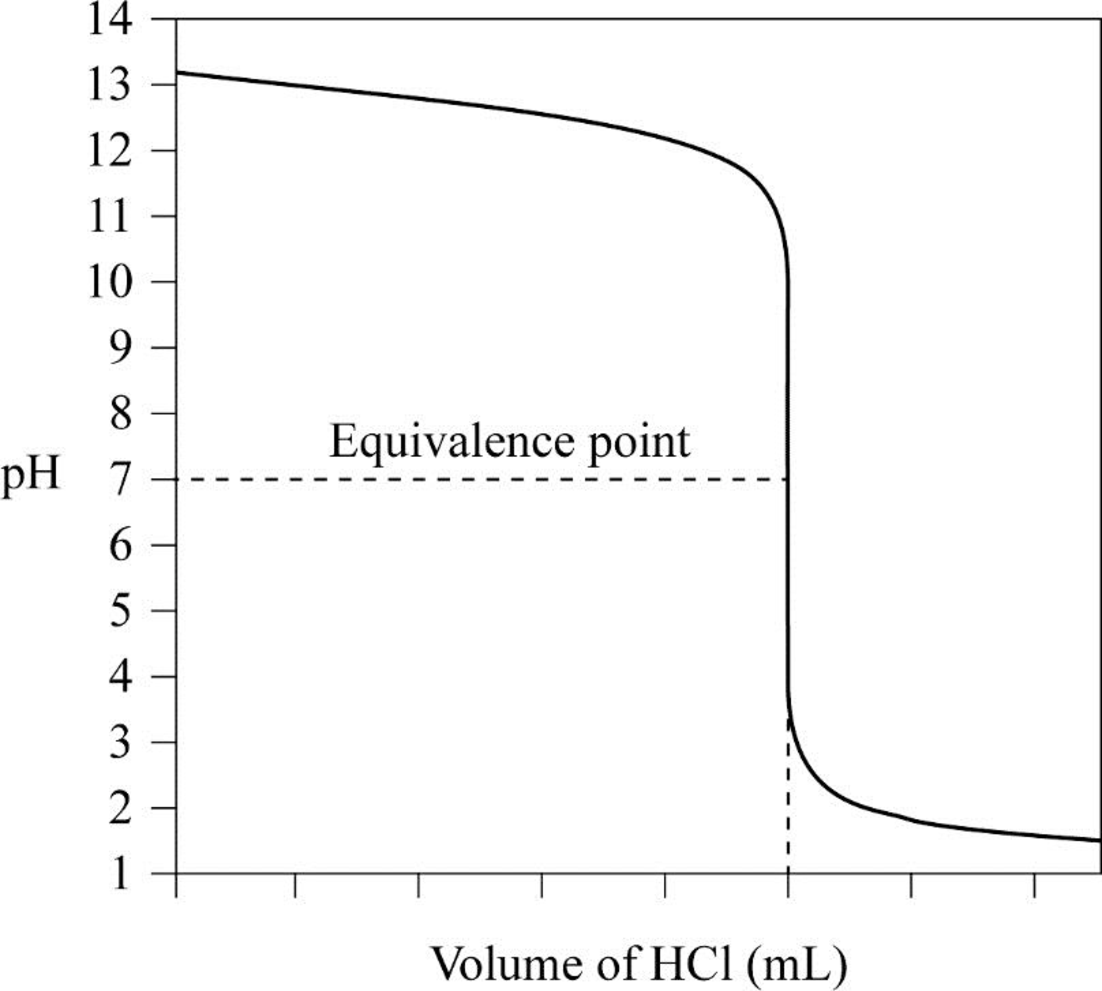
From the result titration curve has to be plotted and position of the equivalent point has to be indicated.
(g)
Explanation of Solution
Table for the volume of
| S.No | Volume of | |
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 |
The equivalent point of the given solution is seven when the volume of
From the result, the titration curve is plotted and position of the equivalent point is indicated as follows.
Want to see more full solutions like this?
Chapter 15 Solutions
Chemistry: The Molecular Science, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- Vitamin C is ascorbic acid (HC6H7O6), for which Ka is 8.0105. Calculate the pH of a solution made by dissolvinga 500-mg tablet of pure vitamin C in water anddiluting to 100 mL.arrow_forwardConsider citric acid, H3C6H5O7, added to many soft drinks. The equilibrium constants for its step-wise ionization areKa1=7.5104 ,Ka2=1.7105, andKa3=4.0107. Write the overall net ionic equation and calculate K for the complete ionization of citric acid.arrow_forwardThe pH of 0.259 M solution of an unknown acid is measured to be 2.353. What is the Ka of the acid?arrow_forward
- If a buffer solution is 0.180 M in a weak base (Kb=7.5×10−5) and 0.400 M in its conjugate acid, what is the pH?arrow_forwardIf titrating 25.00 mL of 0.115 M HCl with 0.0955 M NaOH, what is the pH of the solution after 10.48 mL of NaOH has been added?arrow_forwardThe pH of a solution that contains 1.2M acetic acid and 0.920M sodium acetate is?arrow_forward
- Addition of the indicator methyl orange to an unknownsolution leads to a yellow color. The addition of bromthymolblue to the same solution also leads to a yellow color.(a) Is the solution acidic, neutral, or basic? (b) What is therange (in whole numbers) of possible pH values for the solution?(c) Is there another indicator you could use to narrowthe range of possible pH values for the solution?arrow_forwardAcetominophen, HC8H8NO2 (MM=151.17 g/mol), is the active ingredient in Tylenol, a common pain reliever. A solution is made by dissolving 6.54g of acetaminophen in enough water to make 250.0 mL of solution. The resulting solution has a pH of 5.24. What is the Ka for acetaminophen?arrow_forwardThe pH of a 0.060 M monophotic acid is 3.44 calculate the K, of the acid?arrow_forward
- A chemist adds a chemical to pure water and there is a 100-fold incre approximation of the new pH value?arrow_forwardhe pH of an aqueous solution of 0.355 M acetylsalicylic acid (aspirin) , HC9H7O4, is .arrow_forwardA 20-year-old student with a coffee addiction had an episode of extreme hyperventilation due to anxiousness for his upcoming final examinations. Upon clinical assessment, his blood result had a pH of 7.53, and the bicarbonate ion concentration was at 22 mmol/L. What is the molar concentration of the bicarbonate ion? What is the molar concentration of the carbonic acid in mmol/L?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning



