
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.13D, Problem 15.22P
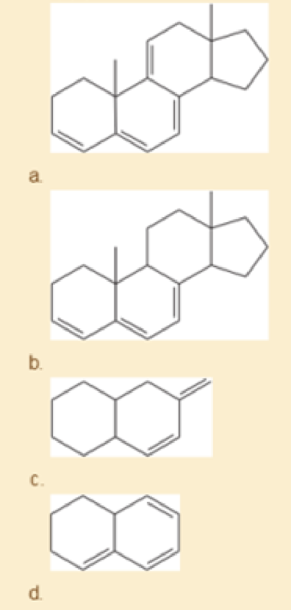
Using the examples in Table15-2 to guide you, match four of the following UV absorption maxima (λmax) with the corresponding compounds: (1) 232 nm; (2) 256 nm; (3) 273 nm; (4) 292 nm; (5) 313 nm; (6) 353 nm.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the following answer using the data provided here:
Molecular formula: C4H8O2
Important IR data (cm-1): 3280 (broad); 2980-2730 (many); 1690; 1254
All 1H NMR data (ppm, splitting, integration): 11.0 ppm (s), 1; 2.57 ppm (m), 1; 1.07 ppm (d), 6
1. Sketch out a 1H NMR spectrum showing peak locations and peak splitting on a ppm scale for the data provided above. Include the data labels above in your spectrum, but do not show integration lines.
2. draw the most proper line bond structure for the data given above
Calculate the IHD of C7H6XNO and identify the important peaks in the following MS spectral data and draw the structure of the important peaks in the following MS spectral data.
A compound has strong infrared absorptions at the following frequencie . Suggest likely functional group that may be present.
Q.) 1710 and 2500–3400 (broad) cm-1
Chapter 15 Solutions
Organic Chemistry (9th Edition)
Ch. 15.2 - Prob. 15.1PCh. 15.2 - Prob. 15.2PCh. 15.2 - Prob. 15.3PCh. 15.4 - Prob. 15.4PCh. 15.4 - Prob. 15.5PCh. 15.5 - Treatment of an alkyl halide with AgNO3 in alcohol...Ch. 15.5 - Propose a mechanism for each reaction, showing...Ch. 15.6 - When Br2 is added to buta-1,3-diene at 15 C, the...Ch. 15.7 - Prob. 15.9PCh. 15.7 - When N-bromosuccinimide is added to hex-1-ene in...
Ch. 15.7 - Prob. 15.11PCh. 15.9 - Addition of 1-bromobut-2-ene to magnesium metal in...Ch. 15.10 - Show how you might synthesize the following...Ch. 15.11 - Predict the products of the following proposed...Ch. 15.11 - Prob. 15.15PCh. 15.11A - Prob. 15.16PCh. 15.11B - Prob. 15.17PCh. 15.11B - Predict the products of the following Diels-Alder...Ch. 15.12C - Prob. 15.19PCh. 15.12C - Prob. 15.20PCh. 15.13C - Prob. 15.21PCh. 15.13D - Using the examples in Table15-2 to guide you,...Ch. 15.14 - Phenolphthalein is an acid-base indicator that is...Ch. 15 - Prob. 15.24SPCh. 15 - Prob. 15.25SPCh. 15 - Show how the reaction of an allylic halide with a...Ch. 15 - Prob. 15.27SPCh. 15 - A solution was prepared using 0.0010 g of an...Ch. 15 - Prob. 15.29SPCh. 15 - Prob. 15.30SPCh. 15 - Prob. 15.31SPCh. 15 - Prob. 15.32SPCh. 15 - Prob. 15.33SPCh. 15 - Give the structures of the products represented by...Ch. 15 - Furan and malemide undergo a Diels-Alder reaction...Ch. 15 - Prob. 15.36SPCh. 15 - Prob. 15.37SPCh. 15 - Prob. 15.38SPCh. 15 - Prob. 15.39SPCh. 15 - Determine whether each structure is likely to be...Ch. 15 - An important variation of the Diels-Alder reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The mass spectrum (a) and the infrared spectrum (b) of an unknown hydrocarbon are shown. Propose as many structures as you can.arrow_forwardPropose a structure consistent with each set of data. C8H10 : IR absorptions at 3108–2875, 1606, and 1496 cm−1arrow_forwardUsing the attached infrared spectroscopy spectrum for the compound Tetrahydrofuran, identify the functional group and any observed characteristic absorbtions in cm-1. Compare with the attached roadmap for interpreting IR spectra.arrow_forward
- What functional group can you get from its IR spectrum? Support your answer by identifying prominent peaks (wavenumbers cm^-1) and assigning the functional group.arrow_forwardPredict the characteristic infrared absorptions of the functional groups in the following molecules. ) pent-1-ynearrow_forwardWhich of the following compounds will show two absorption bands at around 3300 cm-1 and around 2150 cm-¹?arrow_forward
- 2 The IR and 1H-NMR spectra of a compound with molecular formula C4H7ClO2 are shown below. Your objective as a group is to propose a structure for this compound, explaining how you reach your decision. Using all the information you have been given, in a post with others in your group share your initial ideas about the possible structure of the compound. Then use comments to interact with the other students in the group and propose a final answer to the problem. In the comment phase, you should comment on the postings of at least two other students.arrow_forwardWhat is the structure of the unknown? Determine it by analyzing the spectroscopic data given in IR, MS, 1H NMR, 13C NMR (Broadband decoupled with carbon types) and fully substantiate your answer with key supporting data from each spectrum.arrow_forwardN-propylbenzene, C6H5CH2CH3, contains C (sp3) -H and C (sp2) -H bonds. Its IR spectrum shows strong or medium absorptions at 3085, 3064, 3028, 2960, 2931 and 2873 cm ^ -1, as well as bands below 1600cm -1. Which statement is wrong? A.) Stretching of the C (sp3) -H bonds results in absorptions at lower wave numbers than the stretching of the C (sp2) -H bonds. B.) The absorptions at 2960, 2931 and 2873 cm ^ -1 are assigned to stretching of the C (sp3) -H bonds. C.) The absorptions at 3085, 3064 and 3028 cm ^ -1they are assigned to stretching of the C (sp2) -H bonds. D.) Each absorption can be assigned to the stretch mode of a particular bond in the propylbenzene molecule.arrow_forward
- I need help with questions please show on charts, please information Functional group- primary amine Derivatives - Benzamide unknown compound C3H9N 1: Label identifiable absorption above 1400 cm-1, sp2 carbon stretch foralkenes and aromatic, sp3 carbon-hydrogen stretch, sp2 carbon-hydrogenstretch, carbon hydrogen stretches of aldehyde, O-H stretch for alcohols, andcarboxylic acid, C=O stretches, N-H for amines.2- 1HNMR: Draw the structure neatly on the spectrum with each of the hydrogenclearly labelled. Identify which signal is associated with each type of H asmuch as possible.3- 13CNMR: Draw the structure neatly on the spectrum with each of the carbonclearly labelled. Identify which signal is associated with each type of carbonas much as possiblearrow_forwardCalculate the index of hydrogen deficiency of this compound Q.)Pyridine, C5H5Narrow_forwardWrite notes on the most important absorptions and fragmentations found in the following spectra and use the information to suggest the best possible structure. (Molecular formula: C8H1602, M =144)...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY