
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.14, Problem 15.23P
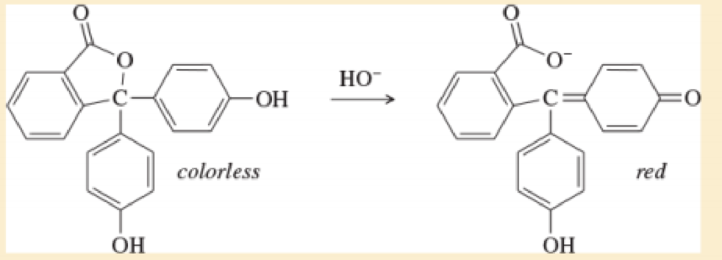
Phenolphthalein is an acid-base indicator that is colorless below pH 8 and red above pH 8. Explain briefly why the first structure is colorless and the second structure is colored.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Phenolphthalein is an acid–base indicator. In solutions of pH < 8.5, it is colorless; in solutions of pH > 8.5, it is deep red-purple. Account for the change in color.
Which acetic acid plus acetate solution has the greatest buffering capacity?
0.335M HC2H3O2 and 0.497 M KC2H3O2
0.520 M HC2H3O2 and 0.116 M NaC2H3O2
0.335M HC2H3O2 and 0.497 M NaC2H3O2
0.820 M HC2H3O2 and 0.715 M NaC2H3O2
0.120 M HC2H3O2 and 0.115 M NaC2H3O2
Explain why the indicator cresol red changes color when the pH is lowered from 10 to 6. What colors will be observed at pH 10, 8, and 6? Why does the color transition require ,2 pH units for completion?
Chapter 15 Solutions
Organic Chemistry (9th Edition)
Ch. 15.2 - Prob. 15.1PCh. 15.2 - Prob. 15.2PCh. 15.2 - Prob. 15.3PCh. 15.4 - Prob. 15.4PCh. 15.4 - Prob. 15.5PCh. 15.5 - Treatment of an alkyl halide with AgNO3 in alcohol...Ch. 15.5 - Propose a mechanism for each reaction, showing...Ch. 15.6 - When Br2 is added to buta-1,3-diene at 15 C, the...Ch. 15.7 - Prob. 15.9PCh. 15.7 - When N-bromosuccinimide is added to hex-1-ene in...
Ch. 15.7 - Prob. 15.11PCh. 15.9 - Addition of 1-bromobut-2-ene to magnesium metal in...Ch. 15.10 - Show how you might synthesize the following...Ch. 15.11 - Predict the products of the following proposed...Ch. 15.11 - Prob. 15.15PCh. 15.11A - Prob. 15.16PCh. 15.11B - Prob. 15.17PCh. 15.11B - Predict the products of the following Diels-Alder...Ch. 15.12C - Prob. 15.19PCh. 15.12C - Prob. 15.20PCh. 15.13C - Prob. 15.21PCh. 15.13D - Using the examples in Table15-2 to guide you,...Ch. 15.14 - Phenolphthalein is an acid-base indicator that is...Ch. 15 - Prob. 15.24SPCh. 15 - Prob. 15.25SPCh. 15 - Show how the reaction of an allylic halide with a...Ch. 15 - Prob. 15.27SPCh. 15 - A solution was prepared using 0.0010 g of an...Ch. 15 - Prob. 15.29SPCh. 15 - Prob. 15.30SPCh. 15 - Prob. 15.31SPCh. 15 - Prob. 15.32SPCh. 15 - Prob. 15.33SPCh. 15 - Give the structures of the products represented by...Ch. 15 - Furan and malemide undergo a Diels-Alder reaction...Ch. 15 - Prob. 15.36SPCh. 15 - Prob. 15.37SPCh. 15 - Prob. 15.38SPCh. 15 - Prob. 15.39SPCh. 15 - Determine whether each structure is likely to be...Ch. 15 - An important variation of the Diels-Alder reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a solution of phenylhydrazine (C6H5-NH-NH2) with ionic strength 0.1 mol/L has pH = 8.13. Determines the pKa for the phenylhydrazinium ion (C6H5-NH-NH3+)arrow_forwardExplain brieflyWhat would happen to the total hardness if the solution was buffered to pH 13.00? What is done to the soda ash solution when the pre-equivalence point of the methyl orange endpoint is reached? What compound is trying to be removed?arrow_forwardHow do the reactions of phenol samples with FeCl3compare? Which structural component of the phenols account for the observation? 2. What compound is the precipitate formed in the Bromine water test? 3. Write the reaction formed in the formation of phenolphthalein. Identify the functional group in phenolphthalein, which is responsible for the indicator property. 4. What is the significance of Millon's test?arrow_forward
- Standard solution : 0.201 grams of acetylsalicylic acid was combined with 10 mL of 0.5 M NaOH, then transfered to a 100 mL flask and filled the rest of the way with water Then 0.500 mLs of the standard solution was transfered to a 10.00 mL flask and diluted to the 10 mL mark with 0.02 M of buffered iron chloride. Number of moles of NaOH = 0.005 moles Since the reaction between acetylsalicylic acid and NaOH is a 1:1 reaction, the number of moles of acetylsalicylic acid used in the reaction is also 0.005 moles. the concentration from analysis question 5: acetylsalicylic acid in the 100.00 mL volumetric flask is 0.050 M. From your plot, what is the value of εb? From the Beer- Lambert law For the aspirin sample, calculate the concentration of acetylsalicylic acid present using the value of εb that you found The concentration from analysis question 5 represents the concentration in the 10.00 mL sample that was prepared in the volumetric flask using an aliquot of the solution in the…arrow_forwardPhenolphthalein is an acid–base indicator. In solutions of pH 6 8.5, it is colorless; in solutions of pH 7 8.5, it is deep red-purple. Account for thechange in color.arrow_forwardPlease answer the following questions: 1. The phenolphthalein HIn indicator forms this equilibrium in an aqueous solution: HIn + H2O ↔ H3O+ + In-. During the course of titrating the acid content of samples, the concentration of H3O+ _________ , shifting the equilibrium towards _________ , until end-point is reached. a.decreases, In- b.increases, HIn c.decreases, HIn d.increases, In- 2. In the distillation of 70% isopropyl alcohol, which of the following is TRUE? a.The temperature was maintained at the boiling point of water. b.The 20 mL sample was distilled to dryness. c.The thermometer was allowed to touch the solution in the distilling flask. d.None of the above 3. Some Chem 16.1 students performed the ideal gas experiment on a hot summer day. Upon doing their experiment, the recorded temperature was 302 K.The pressure of the trapped air was found to be 763.0 torr. Given the following vapor pressures of water at certain temperatures, calculate the pressure of…arrow_forward
- On the basis of Le Chatelier's principle, explain why Ag2CO3 dissolves when HNO3 is added.arrow_forwardThe Ka of bromoacetic acid is 2.00 ×10–3. What masses of bromoacetic acid (CH2BrCOOH) and sodium bromoacetate (CH2BrCOONa) are needed to prepare 1.00 L of pH = 1.700 buffer if the total concentration of the two components is 0.200 M?arrow_forwardWhy phenolphthalein is a suitable indicator in the titration of a strong acid with a strong base?arrow_forward
- In two SEPARATE flasks containing similar component of carbonate mixtures, the following volumes of titrant were obtained for phenolphthalein and methyl orange endpoin respectively: (1) 21.0mL (2) 24.0mL. What is/are the component/s of the mixture that is/are being reacted at the PHENOLPHTHALEIN endpoint? a. NaOH and NaHCO3 b. Na2CO3 only c. NaOH and Na2CO3 d. NaHCO3 onlyarrow_forwardA. What two compounds would be best to use in preparing a pH 9.0 buffer solution? justify your answer. Assume you have the acids and the sodium salts of their conjugate bases available to you. Answer: The two compounds best fo preparing a buffer solution with a pH of 9.0 would be hydrocyanic (HCN) and phenol (HOc6H5) because their pKa values are 9.40 and 9.80 respectively making their pH levels the closest to 9. B. WHat mass ration of sodium to acid is needed to prepare this buffer? C. Can this buffer solution better resist pH changes from a string acid addition or strong base addition?arrow_forwardWhy phenolphthalein is not a suitable indicator in the titration of a strong acid with a weak base?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY