
Liquid octane (C8H18) enters a steady-flow combustion chamber at 25°C and 1 atm at a rate of 0.25 kg/min. It is burned with 50 percent excess air that also enters at 25°C and 1 atm. After combustion, the products are allowed to cool to 25°C. Assuming complete combustion and that all the H2O in the products is in liquid form, determine (a) the heat transfer rate from the combustion chamber, (b) the entropy generation rate, and (c) the exergy destruction rate. Assume that T0 = 298 K and the products leave the combustion chamber at 1 atm pressure.
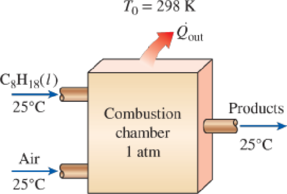
FIGURE P15–87
(a)
The rate of heat transfer from the combustion chamber.
Answer to Problem 83P
The rate of heat transfer from the combustion chamber is
Explanation of Solution
Write the energy balance equation using steady-flow equation.
Here, the total energy entering the system is
Substitute
Here, the enthalpy of formation for product is
Calculate the molar mass of the
Here, the number of carbon atoms is
Determine the rate of mole flow rates of the product.
Here, the mass flow rate is
Determine the heat transfer rate from the combustion chamber.
Conclusion:
Write the theoretical combustion equation of for
Here, liquid octane is
Calculate the stoichiometric coefficient of air by
Substitute
From the Table-26, “Enthalpy of formation, Gibbs function of formation, and absolute entropy at
| Substance | |
| -249,950 | |
| 0 | |
| 0 | |
| -285,830 | |
| -393,520 |
Refer Equation (VII), and write the number of moles of reactants.
Here, number of moles of reactant octane, oxygen and nitrogen is
Refer Equation (VII), and write the number of moles of products.
Here, number of moles of product carbon dioxide, water, oxygen and nitrogen is
Substitute the value table (I) of substance in Equation (II).
Therefore the heat transfer for
Substitute 8 for
Substitute
Substitute
Thus, the rate of heat transfer from the combustion chamber is
(b)
The entropy generation rate from the combustion chamber.
Answer to Problem 83P
The entropy generation rate from the combustion chamber is
Explanation of Solution
Write the expression for entropy generation during this process.
Write the combustion equation of Equation (VI)
Here, the entropy of the product is
Determine the entropy at the partial pressure of the components.
Here, the partial pressure is
Determine the entropy generation rate from the combustion chamber.
Conclusion:
Refer Equation (X) for reactant and product to calculation the entropy in tabular form as:
For reactant entropy,
| Substance |
(T, 1 atm) | ||||
| 1 | 1.00 | 360.79 | --- | 360.79 | |
| 18.75 | 0.21 | 205.14 | -12.98 | 4089.75 | |
| 70.50 | 0.79 | 191.61 | -1.96 | 13646.69 | |
For product entropy,
| Substance |
(T, 1 atm) | ||||
| 8 | 0.0944 | 213.80 | -19.62 | 1867.3 | |
| 9 | --- | 69.92 | --- | 629.3 | |
| 6.25 | 0.0737 | 205.04 | -21.68 | 1417.6 | |
| 70.50 | 0.8319 | 191.61 | -1.53 | 13616.3 | |
Substitute
Substitute
Thus, the entropy generation rate from the combustion chamber is
(c)
The exergy destruction rate from the combustion chamber.
Answer to Problem 83P
The exergy destruction rate from the combustion chamber is
Explanation of Solution
Write the expression for exergy destruction during this process.
Here, the thermodynamic temperature of the surrounding is
Conclusion:
Substitute
Thus, the exergy destruction rate from the combustion chamber is
Want to see more full solutions like this?
Chapter 15 Solutions
Thermodynamics: An Engineering Approach
- Calculate the enthalpy of combustion of propane C3H8 at 25 oC in both kJ/kg and kJ/mole under the following conditions:- 1- gaseous propane with H2O liquid in the products. 2- gaseous propane with H2O vapor in the products 3- liquid propane with H2O liquid in the products 4- liquid propane with H2O vapor in the products note: the enthalpy of evaporation of propane at 25 oC is 425 kJ/kgarrow_forwardOne mole of C3H8, is burned with an unknown amount of air during a combustion process. An analysis of the combustion products shows that the combustion is complete and there 3 moles of free O2, in the products. Determine; - a. the actual air-fuel ration b. the equivalence ratio c. the percentage of theoretical air used during this process.arrow_forwardOne Kmol of C8H18 is burned with 100% air containing 25 Kmol of O2. Determine the air-fuel ratio for this combustion process.arrow_forward
- How do you account for the existence of both complete and incomplete combustion? Explain.arrow_forwardA sample of coal was found to have the following percentage composition C=75 %, H2 = 5.2 %, O2 = 12.8 %, N2 = 1.2 % and the rest ash. Calculate the amount of air needed for the complete combustion if 1 kg of the coal is burnt with 30 % excess air.arrow_forwardDetermine the enthalpy of combustion (in kJ) when fully-consuming a 23-L tank of ethane. The ethane inside the tank is pressurized to 8 atm at 30 ⁰C.arrow_forward
- Propal alcohol (C3H7OH) is burned with 50 percent excess air. Write the balanced reaction equation for complete combustion and determine the air-to-fuel ratio.arrow_forwardA fuel mixture of 60 percent by mass methane (CH4) and 40 percent by mass ethanol (C2H6O), is burned completely with theoretical air. If the total flow rate of the fuel is 10 kg/s, determine the required flow rate of air.arrow_forwardThirty kilograms of butane gas per minute burns with dry air inside a combustion chamber, giving products having the dry molar analysis of 11.0% carbon dioxide, 1.0% carbon monoxide, 3.5% oxygen, and 84.5% nitrogen. Calculate the minimum duct diameter, in mm, used to supply the combustion air at 1 bar and 27 deg C and at a flow velocity of 15 m/s.arrow_forward
- Propane is burned with dry air. A volumetric analysis of the products of combustionreveals the following volume percent composition on a dry basis, 8% CO2, 0.5% CO,7% O2, and 84% N2. Determine the percent of theoretical air used in the combustionprocess.arrow_forwardLiquid propane (C 3 H 8 ) enters a combustion chamber at 25 °C at a rate of 0.05 kg/min where it is mixed and burned with theoretical air that enters the combustion chamber at 7 °C. an analysis of combustion gases reveals that all the hydrogen in the fuel burns to H 2 O but only but only 90% of carbon burn to CO 2 with the remaining 10% forming Co if the exit temperature of combustion gases is 1500 K (a) the mass flow rate of air and (b) the rate of heat transfer from the combustion chamberarrow_forwardAcetylene (C2H2) is burned with the stoichiometric amount of air during a combustion process. Assume complete combustion. Part A Determine the air-fuel ratio on a mass basis. Part B Determine the air-fuel ratio on a mole basis. Part C What-if scenario: What would the air to fuel ratio on a mass basis be if propene (C3H6) was burned instead of acetylene?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





