
To find:
a) Which acid yields a solution with the lower pH if
b) Which acid will dissociate to a greater degree?
c) What is the pH of a solution that contains
d) What is the
Answer to Problem 16.134QA
Solution:
a) Acetoacetic acid
b) Acetoacetic acid
c)
d)
Explanation of Solution
1) Concept:
We are asked to find which acid yields a solution with the lower
2) Formula:
i)
where,
ii)
iii)
3) Given:
β-hydroxybutyric acid,
Acetoacetic acid,
For part a:
Concentration of acetoacetic acid
Concentration of β-hydroxybutyric acid
For part c:
Concentration of acetoacetic acid
Concentration of sodium acetoacetate
For part d:
Concentration of β-hydroxybutyric acid
Concentration of β-hydroxybutyrate anion
Concentration of
Volume of
Molar mass of β-hydroxybutyric acid
Molar mass of β-hydroxybutyrate anion
4) Calculation:
a) Which acid yields a solution with the lower pH if
The
The dissociation reaction for β-hydroxybutyric acid can be written as
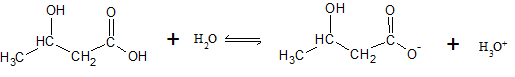
| [β — hydroxybutyric acid] (M) |
[β — hydroxybutyric acid] (M) |
[H3O+] (M) |
|
| Initial (I) | |||
| Change (C) | |||
| Equilibrium (E) |
Assuming
The dissociation reaction for acetoacetic acid can be written as
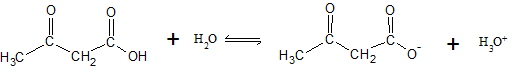
| [Acetoacetic acid] (M) |
[Acetoacetate anion] (M) |
[H3O+] (M) |
|
| Initial (I) | |||
| Change ( C) | |||
| Equilibrium ( E) |
Assuming
b) Which acid will dissociate to a greater degree?
Lower the
c) What is the pH of a solution that contains
The
d) What is the
First we will convert the given concentration from
We assume that the volume of buffer solution is
Calculation of mole of
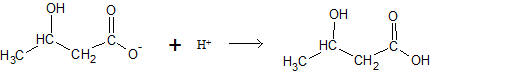
| [A−] (mol) |
[HCL] (mol) |
[HA] (mol) |
|
| Initial | |||
| Change | - |
||
| Final |
The
Conclusion:
Lower the
Want to see more full solutions like this?
Chapter 16 Solutions
CHEMISTRY:ATOMS-FOCUSED..-ACCESS
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





