
(a)
Interpretation:
Balanced titration reaction between
Concept Introduction:
Titration is an analytical method of quantitative chemical analysis which determines the concentration of the analyte. A reagent called titrant is prepared as a standard solution. This titrant reacts with titrand (analyte) solution to determine the concentration of the analyte. The volume of titrant required for titration is termed as the titration volume.
(a)
Explanation of Solution
In this reaction,
(b)
Interpretation:
Two half-reactions for the indicator electrode have to be written.
Concept Introduction:
A
(b)
Explanation of Solution
Complete cell reaction can be classified into oxidation half cell reaction and reduction half cell reaction. In oxidation half, cell oxidation occurs and in reduction half cell reduction occurs. For this case, two half-reactions for the indicator electrode can be written as follows:
Oxidation half cell reaction is as follows:
Reduction half cell reaction is as follows:
(c)
Interpretation:
Two Nernst equations for the cell voltage have to be written.
Concept Introduction:
Nernst equation in
Consider a hypothetical reaction as follows:
For this reaction Nernst equation can be written as follows:
Here,
(c)
Explanation of Solution
Here oxidation half cell reaction is written as follows:
Hence Nernst equation for oxidation half cell at
Here,
Reduction half cell reaction is written as follows:
Hence Nernst equation for reduction half cell at
Here,
(d)
Interpretation:
Electrode potential at
Concept Introduction:
Refer to part (c).
(d)
Explanation of Solution
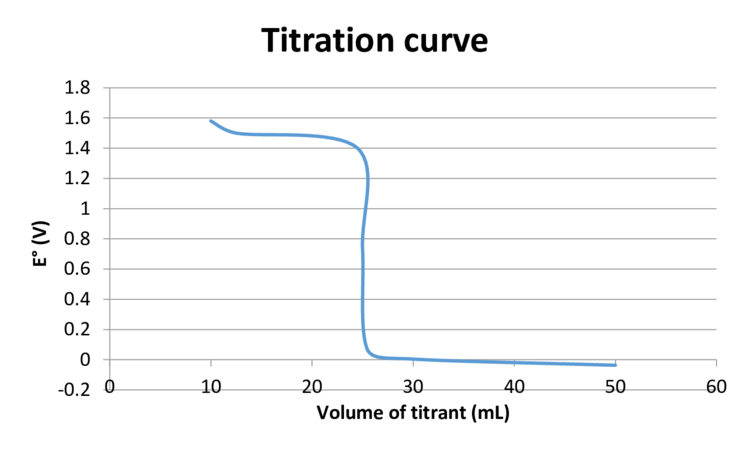
Here titration value of titrant at equivalence point is calculated as follows:
Here titration value of titrant at equivalence point is
When
When
When
When
When
When
The titration curve is as follows:
(e)
Interpretation:
Suitable indicator for the redox titration has to be chosen.
Concept Introduction:
Titration is an analytical method of quantitative chemical analysis which determines the concentration of the analyte. A reagent called titrant is prepared as a standard solution. This titrant reacts with titrand (analyte) solution to determine the concentration of the analyte. The volume of titrant required for titration is termed as the titration volume.
A redox reaction is a type of reaction in which oxidation state of all of the species changes. This type of reaction is actually characterized by the transfer of electron from one species to another.
A redox indicator is a chemical species that undergoes sharp color change at a specific electrode potential. Mostly redox indicators are organic compounds.
(e)
Explanation of Solution
Complete cell reaction can be classified into oxidation half cell reaction and reduction half cell reaction. In oxidation half cell oxidation occurs and in reduction half cell reduction occurs. For this case, two half-reactions for the indicator electrode can be written as follows:
Oxidation half cell reaction is as follows:
Reduction half cell reaction is as follows:
Equivalence point potential for this reaction is
Want to see more full solutions like this?
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





