
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
9th Edition
ISBN: 9780137249442
Author: Wade
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16, Problem 16.40SP
Biphenyl has the following structure.
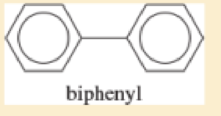
- a. Is biphenyl a (fused) polynuclear aromatic hydrocarbon?
- b. How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?
- c. The heat of hydrogenation for biphenyl is about 418 kJ/mol (100 kcal/mol). Calculate the resonance energy of biphenyl.
- d. Compare the resonance energy of biphenyl with that of naphthalene and with that of two benzene rings. Explain the difference in the resonance energies of naphthalene and biphenyl.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. What is formed when a halogen molecule replaces hydrogen molecule(s) in an aromatic hydrocarbon?
2. What is formed when hydrogen molecules are replaced in an aliphatic hydrocarbon by halogen molecules?
3. What are a class of chemical compounds containing a hydroxyl group (-OH) bonded directly to an aromatic hydrocarbon group (like benzene)?
4. used to test for the presence of aromatic compounds in alcohols?
5. The dehydration of alcohols in the formation of ethers happens at _________.
1. Explain the following
- why stearic acid has higher melting point than decanoic acid.
- why benzoic acid has higher melting point than stearic acid.
- why salicylic acid has higher melting point than benzoic acid.
- why octane has a higher melting point than isooctane.
- why 2,2,3,3-tetramethylbutane has the highest melting point among the three isomers of C8H18.
Benzene’s unusual properties is not limited to hydrogenation. Which one is true about reaction of benzene with Br2?
I. Does not undergo addition reactions typical of other highly unsaturated compounds
II. Benzene does not react with Br2 to yield addition product
III. In the presence of a Lewis acid, bromine substitutes for a hydrogen atom yielding a substitution product
IV. A substitution product still contains a benzene ring
Chapter 16 Solutions
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
Ch. 16.2 - Prob. 16.1PCh. 16.2 - Prob. 16.2PCh. 16.2 - a. Draw the resonance forms of benzene,...Ch. 16.2 - Show the product of the Diels-Alder dimerization...Ch. 16.4 - Prob. 16.5PCh. 16.6 - Make a model of cyclooctatetraene in the tub...Ch. 16.6 - Prob. 16.7PCh. 16.6 - Prob. 16.8PCh. 16.7 - Prob. 16.9PCh. 16.8A - a. Draw the molecular orbitals for the...
Ch. 16.8A - Repeat Problem16-10 for the cyclopentadienyl ions....Ch. 16.8C - Explain why each compound or ion should be...Ch. 16.8C - The following hydrocarbon has an unusually large...Ch. 16.8C - Prob. 16.14PCh. 16.8C - Prob. 16.15PCh. 16.9B - Prob. 16.16PCh. 16.9C - Show which of the nitrogen atoms in purine are...Ch. 16.9C - The proton NMR spectrum of 2-pyridone gives the...Ch. 16.9D - Prob. 16.19PCh. 16.9D - Prob. 16.20PCh. 16.10 - Prob. 16.21PCh. 16.12 - Ciprofloxacin is a member of the fluoroquinolone...Ch. 16.13 - Draw and name all the chlorinated benzenes having...Ch. 16.13 - Name the following compounds:Ch. 16.15 - The UV spectrum of 1-phenylprop-2-en-1-ol shows an...Ch. 16 - Prob. 16.26SPCh. 16 - Name the following compounds:Ch. 16 - Draw and name all the methyl, dimethyl, and...Ch. 16 - Four pairs of compounds are shown. In each pair,...Ch. 16 - One of the following hydrocarbons is much more...Ch. 16 - In Kekuls time cyclohexane was unknown, and there...Ch. 16 - Prob. 16.32SPCh. 16 - Azulene is a deep-blue hydrocarbon with resonance...Ch. 16 - Prob. 16.34SPCh. 16 - Prob. 16.35SPCh. 16 - Prob. 16.36SPCh. 16 - Prob. 16.37SPCh. 16 - Prob. 16.38SPCh. 16 - Prob. 16.39SPCh. 16 - Biphenyl has the following structure. a. Is...Ch. 16 - Anions of hydrocarbons are rare, and dianions of...Ch. 16 - How would you convert the following compounds to...Ch. 16 - Prob. 16.43SPCh. 16 - Prob. 16.44SPCh. 16 - A student found an old bottle labeled thymol on...Ch. 16 - Prob. 16.46SPCh. 16 - Prob. 16.47SPCh. 16 - Prob. 16.48SPCh. 16 - The proton NMR chemical shifts of the hydrogens in...Ch. 16 - Prob. 16.50SPCh. 16 - NMR has been used to probe many molecular...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. What is resonance theory? State five conclusions that can be drawn from the theory. 2. State the two main experiments that were used to establish the extra stability of the benzene molecule? 3. What are the factors that confer aromaticity to an organic molecule? 4. What are the various ways by which alkenes may be synthesized.arrow_forwardWhat type of aromatic compound is pyridine? a. Heterocyclic aromatic compoundb. Benzenoid aromatic compoundc. Non-benzenoid aromatic compoundd. Homonuclear cyclic compoundarrow_forwardWhy do bromine water and potassium permanganate not react with benzene? Will cyclohexene react with bromine and potassium permanganate? If so, give the chemical equation.arrow_forward
- Arrange the following amines in increasing boiling point. O₂N NH₂ NH₂ NH₂ B A O a. Carrow_forwardWhat are the major products of the reaction of ethyl benzoate with hydrochloric acid and water? a. benzoic acid and ethanol b. phenylic acid and ethanol c. ethanoic acid and benzene d. acetic acid and toluene e. phenylic acid and methanolarrow_forwardWhat function does sulfuric acid have in the reaction with 1-butanol to form 1-bromobutane?arrow_forwardExplain why stearic acid has higher melting point than decanoic acid. Explain why salicylic acid has higher melting point than benzoic acid. Explain why benzoic acid has higher melting point than stearic acid. Explain why octane has a higher melting point than isooctane. Explain why 2,2,3,3-tetramethylbutane has the highest melting point among the three isomers of C8H18arrow_forwardOne test for the presence of an alkene is to add a small amount of bromine, which is a red-brown liquid, and look for the disappearance of the red-brown color. This test does not work for detecting the presence of an aromatic hydrocarbon. Explain.arrow_forwardGive two reasons relating to your chemical knowledge why dichlorodimethylsilane is a useful starting material for the formation of methyl silicone polymersarrow_forwardWhat are polycyclic aromatic hydrocarbons (PAHs)?arrow_forwardWhich of these three compounds Alkane , Alkene and Aromatics has an exothermic reaction with concentrated sulfuric acid? Why?arrow_forward1. Octane, C8H18, has 18 different constitutional or chain isomers. One of them, isooctane, is used as a standard in determining the octane rating of gasoline a. Draw the structural formulas for at least ten chain isomers of octane. b. Give the IUPAC name of each. C. Which of the isomers that you have drawn has the highest boiling point? Which has the lowest boiling point? Rationalize. 2. Which of the following structural formulas represent identical compounds and which represent constitutional/structural isomers? Identical compounds: Constitutional isomers: a). CH3CH2CHCH3 e). CH2CH2CHCH3 CH3 i). CH3-C-CI ČI CI CI CH3 CH2CI b). CH3-C-CH3 f). CH3CH2CH2CH,CI j). CICH2 CI CH3 g). CICH,CHCH3 CH2CI k). CH3-CH-CH3 CI c). CH,CHCHCH3 CI h). CH3CHCH2CH2CI CH2CH3 1). CH3CHCI d). CI CIarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY