
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
9th Edition
ISBN: 9780137249442
Author: Wade
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.9C, Problem 16.18P
The proton NMR spectrum of 2-pyridone gives the chemical shifts shown.
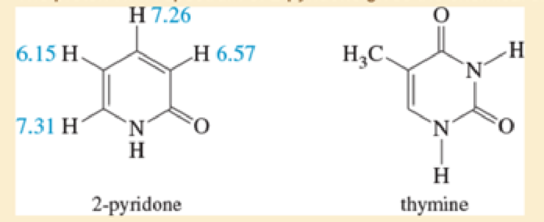
- a. Is 2-pyridone aromatic?
- b. Use resonance forms to explain your answer to (a). Also explain why the protons at δ7.31 and δ7.26 are more deshielded than the other two (δ6.15 and δ6.57).
- c. Thymine is one of the heterocyclic bases found in DNA. Do you expect thymine to be aromatic? Explain.
- d. The structure of 5-fluorouracil is shown in the box at the side of the page. Is 5-fluorouracil aromatic? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Why does H2 not give an IR spectrum?
2. Explain why primary amines and unsubstituted amides have two NH stretching absorptions.
3. Why do anhydrides show two carbonyl peaks?
4. HCl is known to give addition reactions to carbon-carbon double bonds. Why is this behavior not observed in this reaction?
5. Predict the structure of the product expected from addition of molecular bromine to maleic acid.
Step 1: Brief Description
The given compound is 4-aminobenzoic acid. The IR and NMR spectrum of this compound also given.
Determine the spectrum.
Step 2: Determination of IR data
The peaks are identified as following:
Two bands at 3300 & 3230 cm. This is due to asymmetrical and symmetrical N-H stretching of NH₂ group.
A broad stretching between $200-2500 cm** - This broad stretching is due to O-H in carboxylic acid group.
Band at 1680-1700 cm¹. C=O Stretching
1600 cm C-C-C (aromatic carbon)
Step 3: Determination of PMR spectrum
Splitting patterns: It should be noted that spin-spin splitting is observed only between nonequivalent
neighboring protons. Equivalent protons do spin-spin couple with one another but splitting is not
observed.
Chemical shift: A highly shielded proton has a low chemical shift and a highly deshielded proton has a high
value of chemical shift.
The given peaks are of following:
Proton Level
Chemical shift (ppm)
Relative integration
Multiplicity
ABC
-11.9
7.8
8.3…
Aniline (conjugate acid pKa 4.63) is a considerably stronger base than diphenylamine (pKa 0.79). Account for these marked differences.
Chapter 16 Solutions
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
Ch. 16.2 - Prob. 16.1PCh. 16.2 - Prob. 16.2PCh. 16.2 - a. Draw the resonance forms of benzene,...Ch. 16.2 - Show the product of the Diels-Alder dimerization...Ch. 16.4 - Prob. 16.5PCh. 16.6 - Make a model of cyclooctatetraene in the tub...Ch. 16.6 - Prob. 16.7PCh. 16.6 - Prob. 16.8PCh. 16.7 - Prob. 16.9PCh. 16.8A - a. Draw the molecular orbitals for the...
Ch. 16.8A - Repeat Problem16-10 for the cyclopentadienyl ions....Ch. 16.8C - Explain why each compound or ion should be...Ch. 16.8C - The following hydrocarbon has an unusually large...Ch. 16.8C - Prob. 16.14PCh. 16.8C - Prob. 16.15PCh. 16.9B - Prob. 16.16PCh. 16.9C - Show which of the nitrogen atoms in purine are...Ch. 16.9C - The proton NMR spectrum of 2-pyridone gives the...Ch. 16.9D - Prob. 16.19PCh. 16.9D - Prob. 16.20PCh. 16.10 - Prob. 16.21PCh. 16.12 - Ciprofloxacin is a member of the fluoroquinolone...Ch. 16.13 - Draw and name all the chlorinated benzenes having...Ch. 16.13 - Name the following compounds:Ch. 16.15 - The UV spectrum of 1-phenylprop-2-en-1-ol shows an...Ch. 16 - Prob. 16.26SPCh. 16 - Name the following compounds:Ch. 16 - Draw and name all the methyl, dimethyl, and...Ch. 16 - Four pairs of compounds are shown. In each pair,...Ch. 16 - One of the following hydrocarbons is much more...Ch. 16 - In Kekuls time cyclohexane was unknown, and there...Ch. 16 - Prob. 16.32SPCh. 16 - Azulene is a deep-blue hydrocarbon with resonance...Ch. 16 - Prob. 16.34SPCh. 16 - Prob. 16.35SPCh. 16 - Prob. 16.36SPCh. 16 - Prob. 16.37SPCh. 16 - Prob. 16.38SPCh. 16 - Prob. 16.39SPCh. 16 - Biphenyl has the following structure. a. Is...Ch. 16 - Anions of hydrocarbons are rare, and dianions of...Ch. 16 - How would you convert the following compounds to...Ch. 16 - Prob. 16.43SPCh. 16 - Prob. 16.44SPCh. 16 - A student found an old bottle labeled thymol on...Ch. 16 - Prob. 16.46SPCh. 16 - Prob. 16.47SPCh. 16 - Prob. 16.48SPCh. 16 - The proton NMR chemical shifts of the hydrogens in...Ch. 16 - Prob. 16.50SPCh. 16 - NMR has been used to probe many molecular...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. The pk, of vanillin is about 9, which is much more acidic than a normal alcohol. Draw a reaction showing the deprotonation of vanillin with NaOH, and then draw six resonance structures of the conjugate base. Draw the hybrid structure and clearly indicate how the negative charge is distributed in the compound.arrow_forwardThe two following molecules are very common reagents for organic synthesis. They botn have a basic nitrogen, but their pka's are very different (see values given in question 1). The goal of this problem is to understand why. N-H A 1) Please draw below the conjugated acid of A and B respectively: H base conjugated acid base conjugated acid pka of this acid/base pair: - 5 pka of this acid/base pair: ~ 0 2) Both molecules exhibit resonance forms (see below). Draw curved arrows to go from one resonance structure to the next (molecule A: 1->2; molecule B 1->2->3–>4->5). A 1 1 2 4 5arrow_forwardHow does the pKe of the conjugate acid of benzylamine (C3H5CH,NH2) compare to the pK,'s of the conjugate acids of cyclohexanamine (10.7) and aniline (4.6)? Explain your choice.arrow_forward
- Use the four compounds shown below to answer the following questions: a. Why are the ortho-halo-substituted benzoic acids stronger acids than benzoic acid? b. Why is o-fluorobenzoic acid the weakest of the ortho-halo-substituted benzoic acids? c. Why do o-chlorobenzoic acid and o-bromobenzoic acid have similar pKa values?arrow_forwardProvide an explanation without using the pka values : Why is phenol stronger acid than butanoic acid?arrow_forwardHow do the following experimental results support the resonance description of the relative stability of acid chlorides compared to amides? The C–Cl bond lengths in CH3Cl and CH3COCl are identical (178 pm), but the C – N bond inHCONH2 is shorter than the C – N bond in CH3NH2 (135 pm versus 147 pm).arrow_forward
- Pyridine forms a stronger Lewis acid-base complex with SO3 than SO2. However, pyridine forms a weaker complex with SF6 than with SF4. Explain the difference.arrow_forwardDraw the structure of chloric and chlorous acid and predict their pKa values using Pauling's rules.arrow_forward1) Which of the following compounds would have the following peaks on a proton NMR spectrum. a. 2-ethylbenzoic acid b. 4ethylbenzoic acid triplet at 1.75 ppm (3H) quartet at 2.25 ppm (2H) Split Quartet at 7.25 ppm(4H) Singlet at 11.00 ppm (1H) c. 3-ethylbenzoic acid d 3-phenylpropanoic acid.arrow_forward
- 4-Cyanophenol has a pKa = 8.0, whereas phenol has a pKa= 10.0. Resonance form that best illustrates why 4-cyanophenol is more acidic isarrow_forward4-Methylphenol is more acidic than ethanol (pKa 10.36 vs 16.0) , even though both contain an OH group and a methyl group. Draw the structures of the anions formed from loss of the alcoholic protons from both compounds. Use resonance to explain the difference in their respective acidities.arrow_forwardAnalyse the high resolution proton NMR spectrum of a compound with a molecular formula of C8H16O2 and and write its name. Options: A. 2-ethylhexanoic acid B. 1,4-cyclohexanedimethanol C. ethyl hexanoate D. butyl butyrate E. ethyl 2,2-dimethylpropanoatearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY