
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
9th Edition
ISBN: 9780137249442
Author: Wade
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.51SP
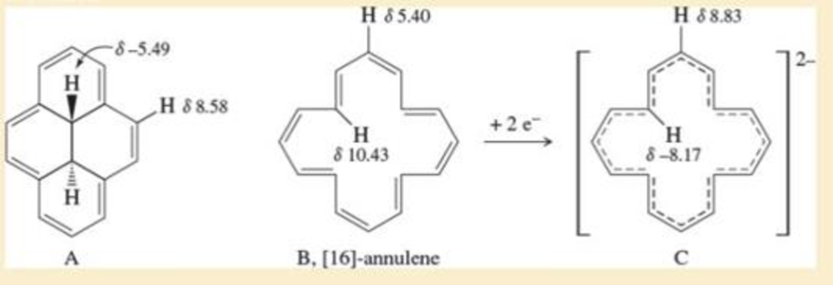
NMR has been used to probe many molecular properties, including aromaticity. One of the interesting electronic effects of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Following are 'H-NMR and BC-NMR spectral data for compound G (CH0). From
this information, deduce the structure of compound G.
'H-NMR
13C-NMR
2.50 (t, 2H)
210.19 126.82
3.05 (t, 2H)
136.64 126.75
3.58 (s, 2H)
133.25
45.02
7.1-7.3 (m, 4H)
128.14
38.11
127.75
28.34
When an NMR spectrum is taken of the molecule below, there is the expected 5H in the
aromatic region, triplet (3H) and quartet (2H) upfield of the aromatic region.
After adding a drop of acidic D₂O, the spectrum changes over a few minutes to one that just
has 5H aromatic protons and an upfield 3H singlet. What happens? Explain with mechanisms.
H3C.
Draw the structure of the compound identified by the simulated 'H NMR and ¹3C NMR spectra.
The molecular formula of the compound is C₁0H₁2O.
(Blue numbers next to the lines in the 'H NMR spectra indicate the integration values.)
¹H NMR
1H
2H
2H
2H
2H
3H
IT 111
10
8
6
8 (ppm)
13C NMR
III.
220
200
180
160
140
60
40
20
100
8 (ppm)
Deduce the structure from the spectra.
Select
Draw
More
CHO
2
Ć
Rings
120
80
Erase
Q 2 Q
Chapter 16 Solutions
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
Ch. 16.2 - Prob. 16.1PCh. 16.2 - Prob. 16.2PCh. 16.2 - a. Draw the resonance forms of benzene,...Ch. 16.2 - Show the product of the Diels-Alder dimerization...Ch. 16.4 - Prob. 16.5PCh. 16.6 - Make a model of cyclooctatetraene in the tub...Ch. 16.6 - Prob. 16.7PCh. 16.6 - Prob. 16.8PCh. 16.7 - Prob. 16.9PCh. 16.8A - a. Draw the molecular orbitals for the...
Ch. 16.8A - Repeat Problem16-10 for the cyclopentadienyl ions....Ch. 16.8C - Explain why each compound or ion should be...Ch. 16.8C - The following hydrocarbon has an unusually large...Ch. 16.8C - Prob. 16.14PCh. 16.8C - Prob. 16.15PCh. 16.9B - Prob. 16.16PCh. 16.9C - Show which of the nitrogen atoms in purine are...Ch. 16.9C - The proton NMR spectrum of 2-pyridone gives the...Ch. 16.9D - Prob. 16.19PCh. 16.9D - Prob. 16.20PCh. 16.10 - Prob. 16.21PCh. 16.12 - Ciprofloxacin is a member of the fluoroquinolone...Ch. 16.13 - Draw and name all the chlorinated benzenes having...Ch. 16.13 - Name the following compounds:Ch. 16.15 - The UV spectrum of 1-phenylprop-2-en-1-ol shows an...Ch. 16 - Prob. 16.26SPCh. 16 - Name the following compounds:Ch. 16 - Draw and name all the methyl, dimethyl, and...Ch. 16 - Four pairs of compounds are shown. In each pair,...Ch. 16 - One of the following hydrocarbons is much more...Ch. 16 - In Kekuls time cyclohexane was unknown, and there...Ch. 16 - Prob. 16.32SPCh. 16 - Azulene is a deep-blue hydrocarbon with resonance...Ch. 16 - Prob. 16.34SPCh. 16 - Prob. 16.35SPCh. 16 - Prob. 16.36SPCh. 16 - Prob. 16.37SPCh. 16 - Prob. 16.38SPCh. 16 - Prob. 16.39SPCh. 16 - Biphenyl has the following structure. a. Is...Ch. 16 - Anions of hydrocarbons are rare, and dianions of...Ch. 16 - How would you convert the following compounds to...Ch. 16 - Prob. 16.43SPCh. 16 - Prob. 16.44SPCh. 16 - A student found an old bottle labeled thymol on...Ch. 16 - Prob. 16.46SPCh. 16 - Prob. 16.47SPCh. 16 - Prob. 16.48SPCh. 16 - The proton NMR chemical shifts of the hydrogens in...Ch. 16 - Prob. 16.50SPCh. 16 - NMR has been used to probe many molecular...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1, 6-Methanonaphthalene has an interesting 1H NMR spectrum in which the eight hydrogens around the perimeter absorb at 6.9 to 7.3 δ, while the two CH2 protons absorb at -0.5 δ. Tell whether it is aromatic, and explain its NMR spectrum.arrow_forwardAlthough benzene itself absorbs at 128 ppm in its 13C NMR spectrum, the carbons of substituted benzenes absorb either upfield or downfield from this value depending on the substituent. Explain the observed values for the carbon ortho to the given substituent in the monosubstituted benzene derivatives X and Y.arrow_forwardUse the observed 1H NMR data to decide whether C and its dianion are aromatic, antiaromatic, or not aromatic. C shows NMR signals at –4.25 (6 H) and 8.14–8.67 (10 H) ppm. The dianion of C shows NMR signals at –3 (10 H) and 21 (6 H) ppm. Why are the signals shifted upeld (or downeld) to such a large extent?arrow_forward
- Construct a structure of the given 1H NMR data signals.A. C5H12O 0.91 δ (3H, triplet) 1.19 δ (6H, singlet) 1.50 δ (2H, quartet) 2.24 δ (1H, singlet) B. C4H10O 0.90 δ (6H, doublet) 1.76 δ (1H, multiplet) 3.38 δ (2H, doublet) 3.92 δ (1H, singlet) C. C5H10O 1.09 δ (6H, doublet) 2.12 δ (3H, singlet) 2.58 δ (1H, septet)arrow_forwardIn the presence of a small amount of acid, a solution of acetaldehyde (CH3CHO) in methanol (CH3OH) was allowed to stand and a new compound L was formed. L has a molecular ion in its mass spectrum at 90 and IR absorptions at 2992 and 2941 cm−1. L shows three signals in its 13C NMR at 19, 52, and 101 ppm. The 1H NMR spectrum of L is given below. What is the structure of L?arrow_forwardThere is a Compound called A with the formula C13H20N2O2. In the proton NMR two triplets at 2.8 and 4.3 are coupled to each other. Similarly, a triplet at 1.1 and a quartet at 2.6 are coupled to each other. the carbonyl group appearing at 1669 cm -1 in the IR spectrum has an unusually low value. Please provide the structure of A.arrow_forward
- Based on the 13C NMR data provided with multiplicity, deduce the structures of each of the benzenoid aromatic compounds below, where the molecular formula for compound is C10H14. a. 134(s), 131(d), 19(q)arrow_forwardFind how many peaks will appear in the proton (1H) NMR spectrum and carbon (13C) NMR spectrum of each molecue. Don't take into account splitting in the proton(1H) NMRarrow_forwardA and B, isomers of molecular formula C3H5Cl3, are formed by the radical chlorination of a dihalide C of molecular formula C3H6Cl2. a.Identify the structures of A and B from the following 1H NMR data: Compound A: singlet at 2.23 and singlet at 4.04 ppm Compound B: doublet at 1.69, multiplet at 4.34, and doublet at 5.85 ppm b.What is the structure of C?arrow_forward
- 1) Which of the following compounds would have the following peaks on a proton NMR spectrum. a. 2-ethylbenzoic acid b. 4ethylbenzoic acid triplet at 1.75 ppm (3H) quartet at 2.25 ppm (2H) Split Quartet at 7.25 ppm(4H) Singlet at 11.00 ppm (1H) c. 3-ethylbenzoic acid d 3-phenylpropanoic acid.arrow_forwardCompound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The 1H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively. - Identify compound B, explaining your reasoningarrow_forwardPropose a structure for an unknown compound whole molecular formula is C(5)H(10)O(2) and is consistent with the 1H NMR data listed. doublet, at 1.23 ppm (6H) singlet, at 2.10 ppm (3H) septet, at 4.98 ppm (1H)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License