
(a)
Interpretation:
The given organic compound has to be named.
Concept introduction:
In chemistry Structure is the arrangement of
IUPAC rules for naming
Name the longest chain that contains the double bond or double bonds. The name of the chain will end in –ene.
Number longest chain so
Name and number the substituent(s) before the name of the longest continuous chain.
Write the alkyl groups in alphabetical order regardless of their position on the chain.
(b)
Interpretation:
The given organic compound has to be named.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
IUPAC rules for naming alcohols
The nomenclature of alcohol like
The position of the alkyl and hydroxyl groups that attached to the carbon chains are shown by numbering the carbon atoms. The hydroxyl group must always get the smallest possible number.
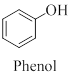
(c)
Interpretation:
The given organic compound has to be named.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
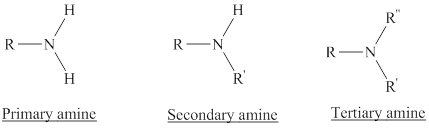
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Fundamentals Of General, Organic, And Biological Chemistry Volume 1 Second Custom Edition For Washington State University, 2/e
- What is the empirical formula of a compound that contains 72.0% carbon, 12.0% hydrogen and 16.0% oxygen by mass?arrow_forwardIf one compound has the formula C5H10 and another has the formula C4H10, are the two compounds isomers? Explain.arrow_forwardBalance the following equation, and tell how many moles of nickel will reactwith 9.81 mol of hydrochloric acid.arrow_forward
- Identify the names of the following structure. (with alpha/beta and L-D designation)arrow_forwardGiven the balanced equation with an unknown compound represented by X, which compound is represented by X?arrow_forwardDraw condensed structural formulas for the two carboxylic acids with the molecular formula C4H8O2arrow_forward
