
Concept explainers
Write the structure of the principal organic product formed in the reaction of
Sulfuric acid (catalytic amount), heat at
°C
Sulfuric acid (catalytic amount), heat at
°C
Dimethyl sulfoxide (DMSO), oxalyl chloride
Pyridinium chlorochromate (PCC) in dichloromethane
Potassium dichromate
Acetic acid 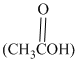 in the presence of dissolved hydrogen chloride
in the presence of dissolved hydrogen chloride
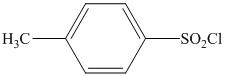 in the presence of pyridine
in the presence of pyridine
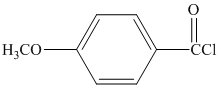 in the presence of pyridine
in the presence of pyridine
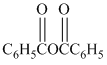 in the presence of pyridine
in the presence of pyridine
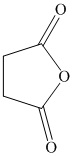 in presence of pyridine
in presence of pyridine
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry - Standalone book
- When 2-pentene is treated with Cl2 in methanol, three products are formed. Account for the formation of each product (you need not explain their relative percentages).arrow_forwardThree isomeric pentanols with unbranched carbon chains exist. Which of these isomers, upon dehydration at 180C, yields only 1-pentene as a product?arrow_forwardAccount for the fact that treating propenoic acid (acrylic acid) with HCl gives only 3-chloropropanoic acid.arrow_forward
- Aldehydes and ketones react with one molecule of an alcohol to form compounds called hemiacetals, in which there is one hydroxyl group and one ether-like group. Reaction of a hemiacetal with a second molecule of alcohol gives an acetal and a molecule of water. We study this reaction in Chapter 16. Draw structural formulas for the hemiacetal and acetal formed from these reagents. The stoichiometry of each reaction is given in the problem.arrow_forwarddescribe how the following conversions could be carried out. In each case give reagents and conditions of the reactions and the structures of the products. a) CH2=CH2 -> CH2 COOH b) ethanol -> 2hydroxypropanoic acidarrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forward
- 4. Heptyl phenyl ketone was formed from the Friedel-Crafts acylation of benzene with an acyl chloride. What is the IUPAC name of the original acyl chloride? 5. How many isomers are there Of C6H14? 6. Aside from a halogenated alkane, what other product results from the bromination of hexane? Write the formulaarrow_forwardCHEMICAL REACTIONS: Illustrate and predict the products of the given chemical reactions below including the correct catalyst for each given reactions Reduction or Hydrogenation of 2-butanone by a metal catalyst.arrow_forwardAlkenes can be hydrated to form alcohols by (1) hydroboration followed by oxidation with alkaline hydrogen peroxide and (2) acid-catalyzed hydration. Compare the product formed from each alkene by sequence (1) with those formed from (2). Q.) cis-2-Butenearrow_forward
- You are given a mixture of benzoic acid, 2-naphthol and 1,4-dimethoxybenzene and you are asked to separate the mixture into its following compounds. You are limited to using dichloromethane, pentane, cyclohexane and ethyl acetate as organic solvents. This doesn't involve solvents used to convert the 3 compounds to ionic salts. Suggest possible organic solvents and explain a procedure you would carry out to separate the 3 mixtures briefly.arrow_forwardIn the reactions involving 1-butanol, 2-butanol and 2-methyl-2-propanol with the formula C4H9OHdescribe what each of the following tests showed about the reactivity of the -OH group and reactions of 1°,2°, and 3° alcohols. The test with neutral KMnO4 The test with concentrated HClarrow_forwardIsoprene has sometimes been used as a starting material in the laboratory synthesis of terpenes. In one such synthesis, the first step is the electrophilic addition of 2 mol of hydrogen bromide to isoprene to give 1,3-dibromo-3-methylbutane.Write a series of equations describing the mechanism of this reaction.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,


