
Concept explainers
(a)
Interpretation:
Show how to bring out the given conversion reaction in good yield.
Concept introduction:
Alkyl or aryl magnesium halides (RMgX) are known as Grignard reagent. The Grignard reaction is an organometallic
Synthesis of Grignard reagent is shown below,
The
Addition of a Grignard reagent to carbon dioxide followed by protonation will produce
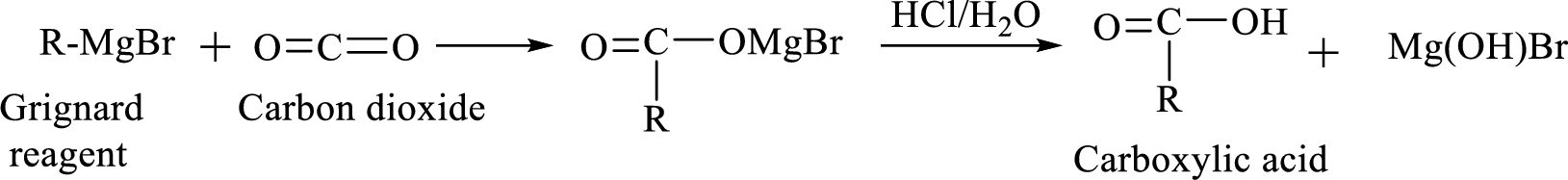
(b)
Interpretation:
Show how to bring out the given conversion reaction in good yield.
Concept introduction:
Carboxylic acid can be prepared from various ways; oxidation of
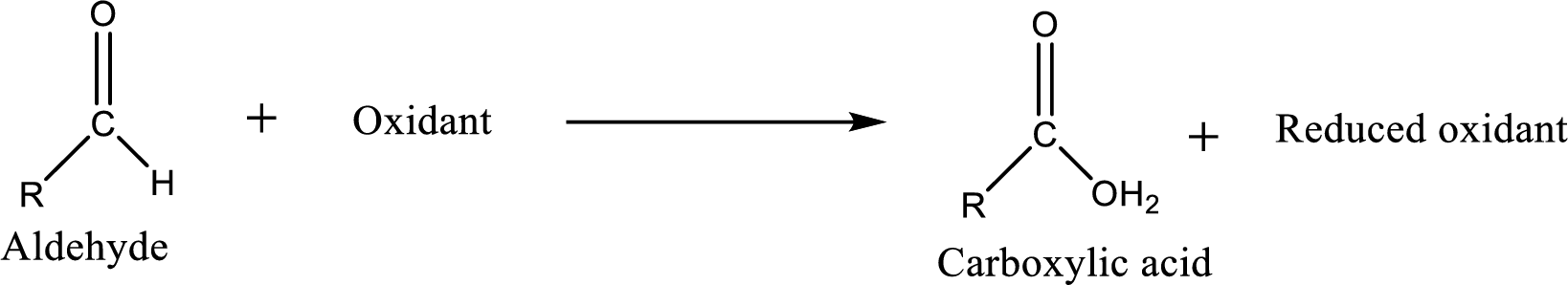
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
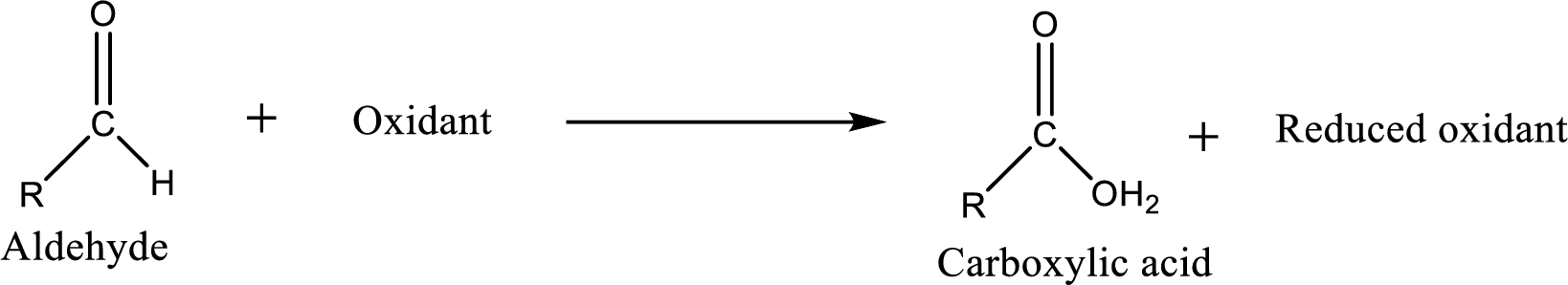
Carboxylic acid on further oxidation removes the carboxyl carbon as carbon dioxide. Depending on the reaction conditions, the oxidation state of the remaining organic structure may be higher, lower or unchanged.
Carboxylic acid can be prepared from primary alcohol by oxidation using strong oxidizing agents like chromic acid,
(c)
Interpretation:
Show how to bring out the given conversion reaction in good yield.
Concept introduction:
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
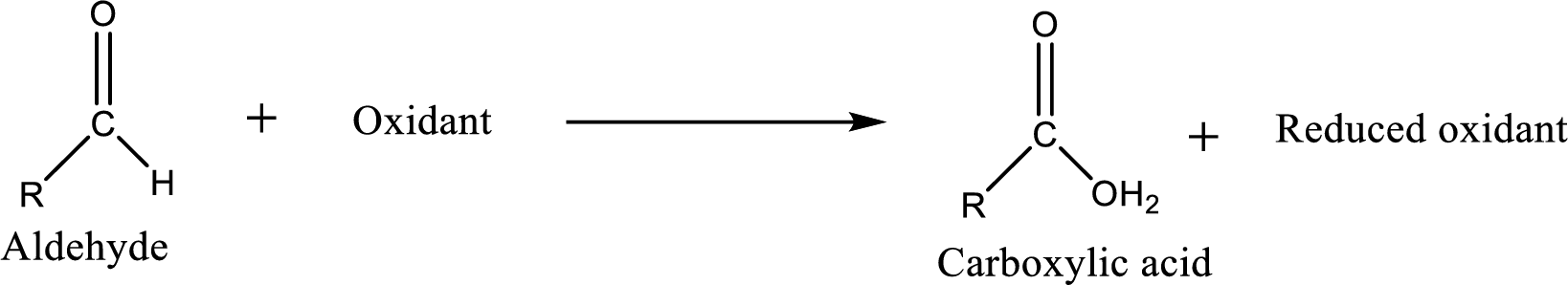
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
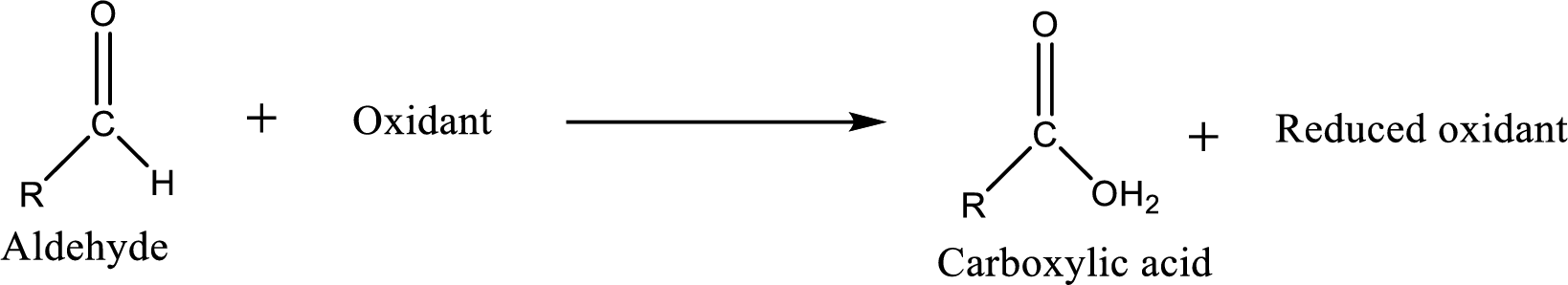
Carboxylic acid on further oxidation removes the carboxyl carbon as carbon dioxide. Depending on the reaction conditions, the oxidation state of the remaining organic structure may be higher, lower or unchanged.
Carboxylic acid can be prepared from primary alcohol by oxidation using strong oxidizing agents like chromic acid,
Trending nowThis is a popular solution!

Chapter 17 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Show how to convert alkenes, alkyl halides, and carbonyl compounds to alcohols.arrow_forwardPart 4: Draw the following molecules. MOST are thiols and phenols. Draw: 3-octanethiol Draw: 2,3-diiodophenol Draw: 1-ethoxy-2-butanethiol Draw: 2-methylbenzenethiol Draw: 1,2-dimethyl 3-chloro-5-propylphenol Draw: 3-cyclobutylphenolarrow_forwardPhenol + Br2/H2Oarrow_forward
- A common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts Select to Draw Select to Draw 1. BH3-THF 1. Hg(OAc)2, Water 2. Н2О2, NaOН 2. NaBHa, NaОНarrow_forwardwrite the missing reactants and products:arrow_forwardDiethyl ether and butan-1-ol are isomers, and they have similar solubilities in water. Their boiling points are very different, however. Explain why these two compounds have similar solubility properties but dramatically different boiling points. CH3CH2¬O¬CH2CH3 CH3CH2CH2CH2¬OH diethyl ether, bp 35 °C butan-1-ol, bp 118 °C 8.4 mL dissolves in 100 mL H2O 9.1 mL dissolves in 100 mL H2Oarrow_forward
- Classify the following reactions as addition (A), substitution (S), elimination (E) or rearrangement (R).arrow_forwardClassify the following organic reactionsarrow_forward2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forward
- Which of the following reactions is a substitution reaction? C6H6 + Cl2 → C6H5Cl + HCl CH2=CH2 + Cl2 → CH2ClCH2Cl CH2=CH2 + HCl → CH3CH2Cl CH2=CH2 + H2 → CH3CH3 HCCH + 2Cl2 → CHCl2CHCl2arrow_forwardFill in the box with the missing reactant,reagents or productarrow_forwardв. Odoriferous Organic Compounds Below each company identify the source or use as recognized by familiar odor. CH3 c-CH-CH,CH,c=CHCH,OH CH3 CH, C-CH-CH,CH,-C-CH-C CH3 CH CH3 H. Geraniol Citral CH 3 С -Н CH3 CH3- CH OCH3 он CH3 CH3 Pinene Carvone Vanillin CH он CH OCH3 CHC-CH ČH,CH-CH2 CH CH3 CH, Menthol Camphor Eugenol C1 co,CH, OH CH=CH-C Methyl salicylate Cinnamaldehyde p-Dichlorobenzenearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
