
(a)
Interpretation:
The principal organic compound that is expected when
Concept introduction:
The addition reaction of the
Answer to Problem 17.23AP
The principal organic compound that is formed when
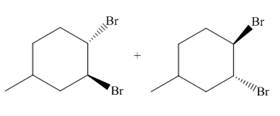
Explanation of Solution
The reaction of
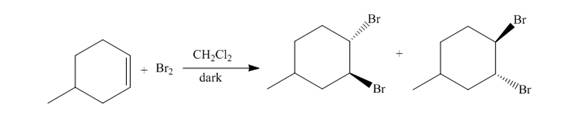
Figure 1
The addition of the bromine molecule on the double bond of the alkene takes place to give the dibromo product. The addition of bromine to the double bond is the anti-addition.
The principal organic compound that is formed when
(b)
Interpretation:
The principal organic compound that is expected when
Concept introduction:
The addition reactions of the alkene are very well known reactions. The electron density on the alkene double bond makes it nucleophilic. The alkene undergoes varieties of addition reaction via different mechanisms.
Answer to Problem 17.23AP
The principal organic compound that is formed when
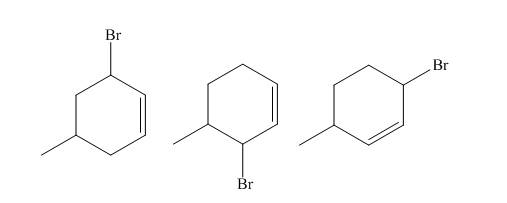
Explanation of Solution
The reaction of
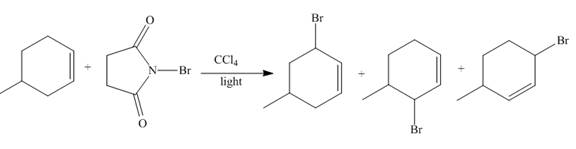
Figure 2
The reaction of an alkene with N-bromosuccinimide in the presence is the free-radical reaction. In this reaction, allylic bromination is observed instead of an addition to the alkene. The N-bromosuccinimide acts as radical initiator. This molecule breaks the
The principal organic compound that is formed when
(c)
Interpretation:
The principal organic compound that is expected when product(s) of part (b) undergo solvolysis in aqueous acetone is to be stated.
Concept introduction:
The nucleophilic substitution reactions are the reactions in which a nucleophile attacks at the electrophilic carbon and eliminates another group. The
The
Answer to Problem 17.23AP
The principal organic compounds that are formed when product(s) of part (b) undergo solvolysis in aqueous acetone are shown below.
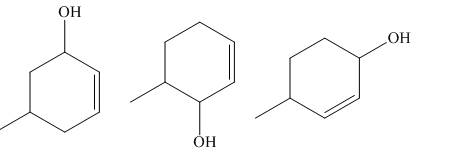
Explanation of Solution
The products of part (b) are shown below.
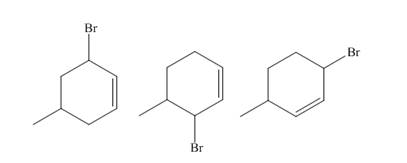
Figure 3
The reaction of product(s) of part (b) on solvolysis in aqueous acetone is shown below.
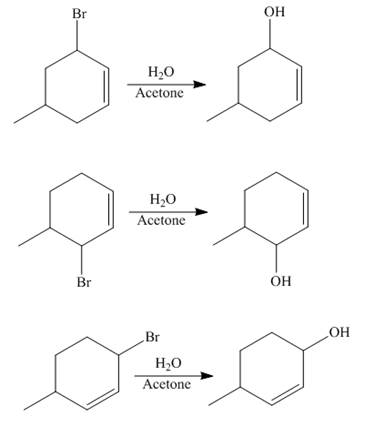
Figure 4
The nucleophilic substitution reaction takes place when solvolysis of products of part (b) is done. The bromine group is substituted by the hydroxyl group giving rise to the formation of an allylic alcohol.
The principal organic compound that is formed when product(s) of part (b) undergo solvolysis in aqueous acetone is shown in Figure 4.
(d)
Interpretation:
The principal organic compound that is expected when product(s) of part (b) are reacted with
Concept introduction:
The reaction of an
Answer to Problem 17.23AP
The principal organic compounds that are formed when product(s) of part (b) are reacted with
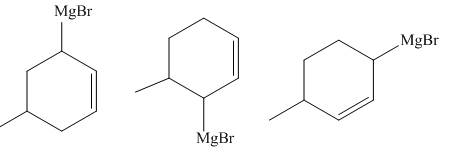
Explanation of Solution
The products of part (b) are shown below.

Figure 3
The reaction that occurs when product(s) of part (b) are reacted with
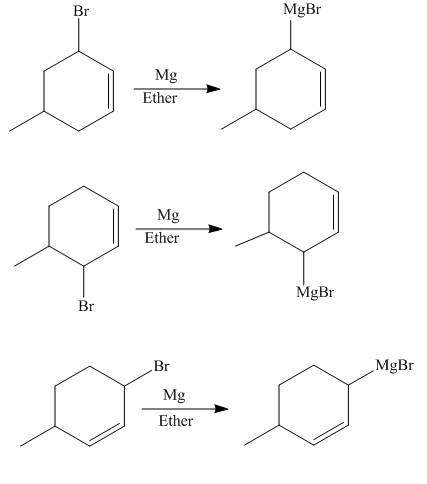
Figure 5
The allylic halide also undergoes the same kind of reactions as the alkyl halide with magnesium metal in dry ether. They also lead to the formation of Grignard reagent but this time with the allylic group.
The principal organic compounds that are formed when product(s) of part (b) are reacted with
(e)
Interpretation:
The principal organic compound that is expected when product(s) of part (d) are reacted with
Concept introduction:
The nucleophilic substitution reactions are the reactions in which a nucleophile attacks at the electrophilic carbon and eliminates another group. The rate of reaction depends upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 17.23AP
The principal organic compounds that are formed when product(s) of part (d) are reacted with
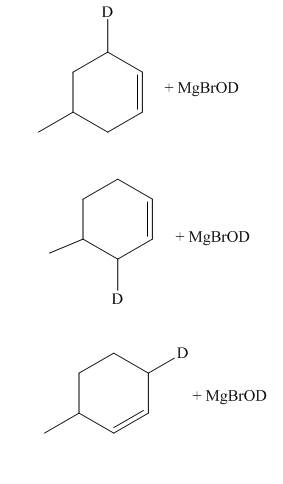
Explanation of Solution
The products of part (d) are shown below.

Figure 6
The reaction of product(s) of part (d) with
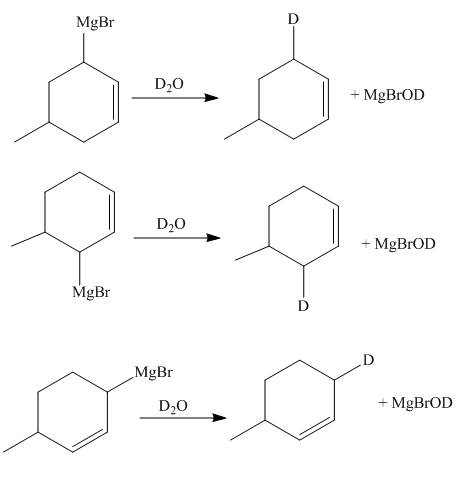
Figure 7
The Grignard reagents are highly reactive towards moisture. The carbon chain on the Grignard reagent gets the hydrogen in place of
The principal organic compounds that are formed when product(s) of part (d) are reacted with
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- -Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardWhat reaction presented in this chapter is occurring in the following equation? Explain the resulting stereochemistry of the reaction.arrow_forwardEthers can often be prepared by SN2 reaction of alkoxide ions, RO*, with alkyl halides. Which of the two possible routes would be the better choice to synthesize the molecule shown below? Explain briefly.arrow_forward
- A hydrocarbon (X), with the molecular formula: C8H14 is reduced in presence of sodium and liquid ammonia to give the only product (Y) with the molecular formula: C8H16. Compounds X and Y both resulting 2,5-dimethylhexane when treated with hydrogen and platinum catalyst (H2/Pt). As a result of the oxidative cleavage of compound Y (by using KMnO4 / H2SO4), a single carboxylic acid derivative with C4H8O2 molecular formula is formed. Again, as a result of the reaction of Y with perbenzoic acid, the chiral compound C8H14O is observed, but the reaction of compound Y with bromine gives the achiral C8H14Br2 as the product.arrow_forwardOzonolysis of compound D will produce compound E and compound F as products. Hydrationof compound D will produce compound G as major product. Compound D is a major productobtained from dehydrohalogenation of 2-chloro-3-methylbutane. a) Identify the structural formula for compound D, E, F and G.b) Suggest suitable reagent(s) and condition(s) needed for dehydrohalogenation of 2-chloro-3-methylbutanearrow_forwardEach of the following reactions has been described in the chemical literature and gives a single organic product in good yield. Identify the product of each reaction.arrow_forward
- Provide the appropriate reagents or product in the following examples..arrow_forwardWhich products are obtained by the following reactions (a) and (b)? Specify the expected main product, if several products can be formed.arrow_forwardDeuterium (D, or 2H) is an isotope of hydrogen with atomic mass=2. Deuterium can be introduced into organic compounds by using reagents in which hydrogen has been replaced by deuterium. Outline preparation of the following compounds from the same alkene using appropriate deuterium-containing reagents.arrow_forward
- What is the major product obtained from treating an excess of each of the following compounds with Cl2 in the presence of ultraviolet light at room temperature? Disregard stereoisomers.arrow_forwardHow many products are obtained following a monochlorination reaction (Cl2, UV) if the stereochemistry is not taken into consideration?arrow_forwardWhat is the major product of the reaction below under the provided thermodynamic conditions?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

