
Concept explainers
(a)
Interpretation:
The product obtained from the reaction of cyclohexene with one equivalent of NBS in
Concept introduction:
NBS stand for
Answer to Problem 17.6P
The product obtained from the reaction of cyclohexene with one equivalent of NBS in
Explanation of Solution
It is known that the
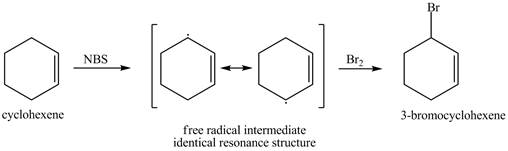
Figure 1
The product obtained from the reaction of cyclohexene with one equivalent of NBS in
(b)
Interpretation:
The products obtained from the reaction of
Concept introduction:
NBS stand for the
Answer to Problem 17.6P
The product obtained from the reaction of
Explanation of Solution
It is known that the
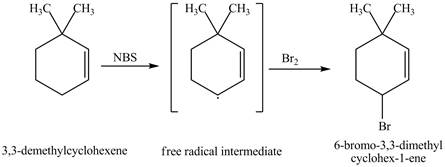
Figure 2
The product obtained from the reaction of
(c)
Interpretation:
The products obtained from the reaction of
Concept introduction:
NBS stand for the
(c)
Answer to Problem 17.6P
The products obtained from the reaction of
Explanation of Solution
It is known that the
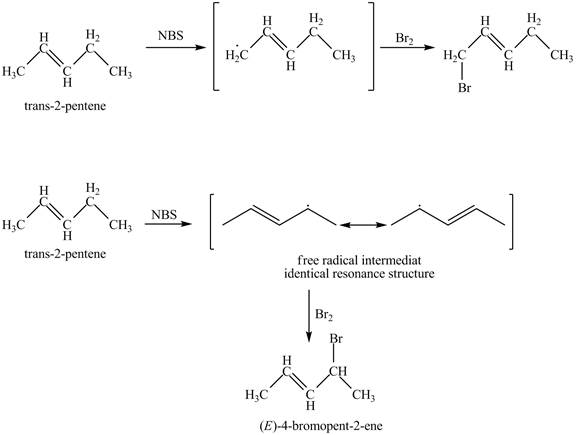
Figure 3
The products obtained from the reaction of
(d)
Interpretation:
The product obtained from the reaction of
Concept introduction:
NBS stand for the
Answer to Problem 17.6P
The product obtained from the reaction of
Explanation of Solution
It is known that the
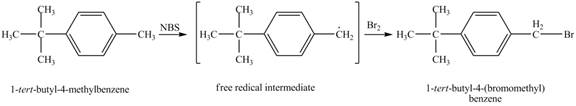
Figure 4
The product obtained from the reaction of
(e)
Interpretation:
The product obtained from the reaction of
Concept introduction:
NBS stand for the
Answer to Problem 17.6P
The product obtained from the reaction of
Explanation of Solution
It is known that the
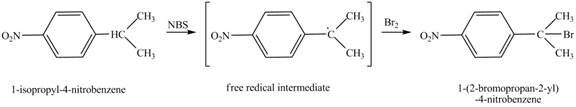
Figure 5
The product obtained from the reaction of
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Following are diastereomers (A) and (B) of 3-bromo-3,4-dimethylhexane. On treatment with sodium ethoxide in ethanol, each gives 3,4-dimethyl-3-hexene as the major product. One diastereomer gives the E alkene, and the other gives the Z alkene. Which diastereomer gives which alkene? Account for the stereoselectivity of each -elimination.arrow_forwardAcid-catalyzed hydrolysis of a 1, 2-epoxycyclohexane produces a transdiaxial 1, 2-diol. What product would you expect to obtain from acidic hydrolysis of cis-3-tert-butyl-l, 2-epoxycyclohexane? (Recall that the bulky tert-butyl group locks the cyclohexane ring into a specific conformation.)arrow_forward3H. Put it all together. Predict the E1 products of the following reaction. Label alkenes as E/Z. 4. Predict the E1 products of the following reaction. Label akenes as E/Z.arrow_forward
- A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardTreatment of p-bromotoluene with NaOH at 300°C yields a mixture of two products, but treatment of m-bromotoluene with NaOH yields a mixture of three products. Explain.arrow_forwardTranexamic acid, a drug useful against blood clotting, is prepared commercially from p-methylbenzonitrile. Formulate the steps likely to be used in the synthesis. (Don’t worry about cis-trans isomers; heating to 300 C interconverts the isomers.)arrow_forward
- The acid-catalyzed dehydration of 1-methylcyclohexanol yields a mixture of two alkenes. How could you use 1H NMR to help you decide which was which?arrow_forwardWhat alkene would yield 2,2-dimethoxycyclopentane-1,3-dicarbaldehyde on treatment with O3 followed by (CH3)2S?arrow_forwardCompound X, C7H14 undergo hydration to form Y which is optically active. X reacts with cold alkane potassium permanganate (VII) solution to form Z. Ozonolysis of X forms methanal and 3,3-dimethylbutanal. Deduce the structure of X,Y and Zarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

