
Concept explainers
Interpretation: A mechanism has to be proposed for the given rearrangement reaction.
Concept introduction:
Benzil-benzilic acid rearrangement reaction:
Benzil-benzilic acid rearrangement reaction is a base catalysed reaction in which,
1,2-diketones are allowed to react with base followed by the acidification to form
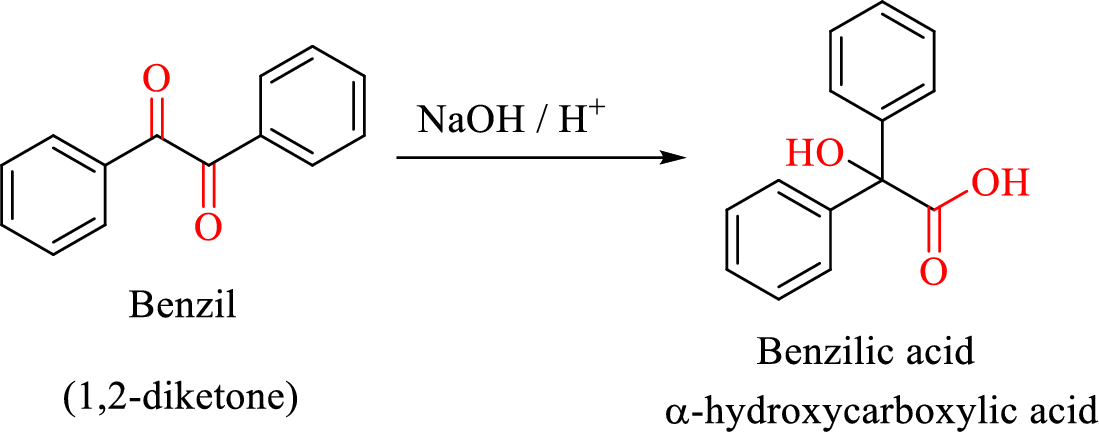
Mechanism of the reaction is the step-by-step description of the process by which reactants are changed into products.
Curved arrows show the bonds that are formed and the bonds that are broken in a reaction.
Curved arrows used to understand a reaction mechanism.
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of
Nucleophile: It is negatively charged species which seeks for positive charge and hence donate pair of electrons to positively charged species (electrophiles) which results in the formation of chemical bond.
Trending nowThis is a popular solution!

Chapter 17 Solutions
OWL V2 with MindTap Reader and Student Solutions Manual eBook for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Claisen condensation between diethyl phthalate and ethyl acetate followed by saponification, acidification, and decarboxylation forms a diketone, C9H6O2. Propose structural formulas for compounds A and B and the diketone.arrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward
- 1 The reaction of an a-diketone with concentrated sodium or potassium hydroxide to give the salt of an a-hydroxyacid is given the general name benzil-benzilic acid rearrangement. It is illustrated by the conversion of benzil to sodium benzilate and then to benzilic acid. Propose a mechanism for this rearrangement. O O НО О Но о H,O Ph—С—С—Рh + NaOH 7 Ph —С—С—O Nat HCI Ph—С—С—ОН H,O Ph Ph Benzil Sodium benzilate Benzilic acid (an a-diketone)arrow_forwardReaction of phenol with acetone in the presence of an acid catalyst gives a compound known as bisphenol A, which is used in the production of epoxy and polycarbonate resins (Section 29.5). Propose a mechanism for the formation of bisphenol Aarrow_forwardPredict the products formed when cyclohexanone reacts with the following reagents. h) sodium acetylide, then mild H3O+arrow_forward
- Barbiturates are prepared by treatment of diethyl malonate or a derivative of diethyl malonate with urea in the presence of sodium ethoxide as a catalyst. Following is an equation for the preparation of barbital, a long-duration hypnotic and sedative, from diethyl diethylmalonate an urea. Barbital is prescribed under one of a dozen or more trade names. Propose a mechanism for This reaction The pKa of barbital is 7.4. Which is the most acidic hydrogen in this molecule and how do you account for its acidity?arrow_forwardBisphenol A is made on a large scale by a condensation of phenol with acetone. Suggest an appropriate catalyst, and propose a mechanism for this reaction.arrow_forwardThe following compound used in perfumery has a violet-like scent. Propose a synthesis of this compound from benzene. 4-Isopropylacetophenonearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

