
(a)
Interpretation:
The preparation method of benzyl methyl ether from toluene is to be stated.
Concept introduction:
Cyclic
Answer to Problem 17.28AP
The preparation method of benzyl methyl ether from toluene is shown below.
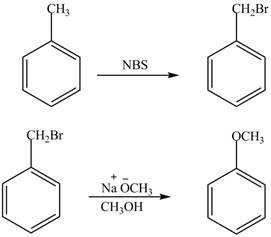
Explanation of Solution
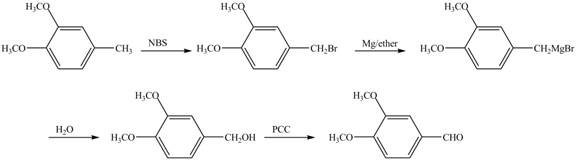
For preparing benzyl methyl ether from toluene, toluene is first brominated.
For bromination of toluene,
The corresponding reaction sequences are shown below.
Figure 1
The preparation method of benzyl methyl ether from toluene is shown in Figure 1.
(b)
Interpretation:
The preparation method of
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of
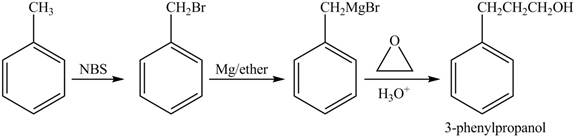
Explanation of Solution
In given reaction, toluene is first brominated with NBS (N-bromosuccinamide). Brominated benzyl reacts with Grignard reagent and
The corresponding reaction sequences to obtain the desired product are shown below.

Figure 2
The preparation method of
(c)
Interpretation:
The preparation method of
Concept introduction:
The Lindlar’s catalyst is composed of
Answer to Problem 17.28AP
The preparation method of
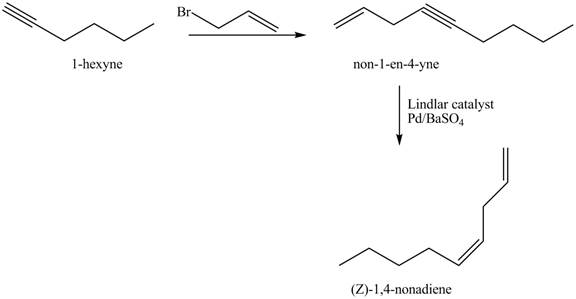
Explanation of Solution
The addition of
The corresponding reaction sequences to obtain the desired product are shown below.
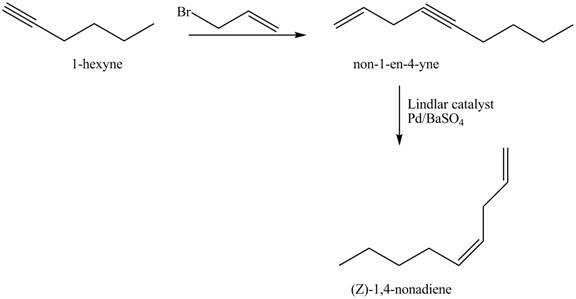
Figure 3
The preparation method of
(d)
Interpretation:
The preparation method of given compound from cyclopentene is to be stated.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of given compound from cyclopentene is shown below.
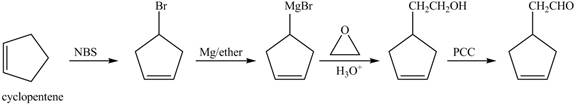
Explanation of Solution
Cyclopentene is first brominated and then on treatment with Grignard reagent and epoxide it gives corresponding alkyl alcohol. This alkyl alcohol is oxidized with mild reagent PCC to convert it into
The corresponding reaction sequences to obtain the desired product are shown below.
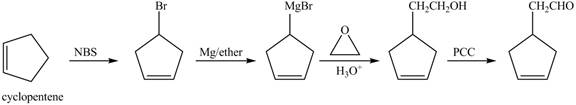
Figure 4
The preparation method of given compound from cyclopentene is shown in Figure 4.
(e)
Interpretation:
The preparation method of
Concept introduction:
The
Answer to Problem 17.28AP
The preparation method of
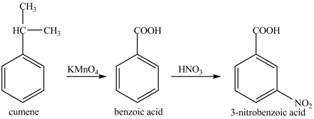
Explanation of Solution
Cumene has to be oxidized first and then its nitration is done to get the required product
Alkyl group is oxidized to gives benzoic acid. This benzoic acid is nitrated with
The corresponding reaction sequences to obtain the desired product are shown below.
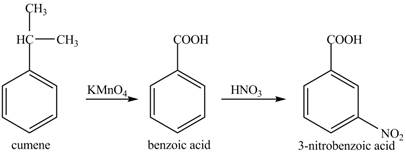
Figure 5
The preparation method of
(f)
Interpretation:
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is to be stated.
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as the electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 17.28AP
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown below.
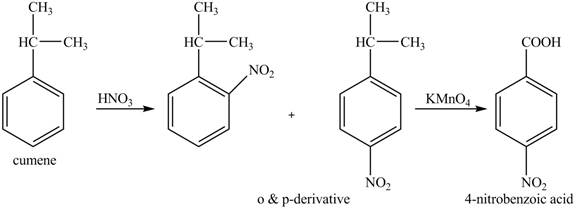
Explanation of Solution
As alkyl group is ortho and para directing, so, both the derivatives are formed. The reaction of cumene with nitric acid results in the formation of ortho and para nitro-cumene. The para nitro cumene is further oxidized to p-nitro benzoic acid with potassium permangnate.
The corresponding reaction sequences to obtain the desired product are shown below.
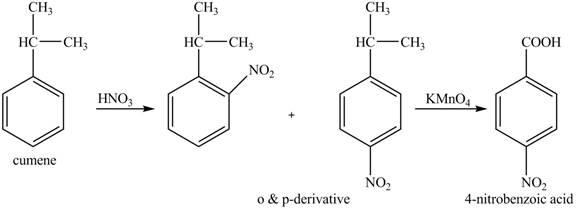
Figure 6
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown in Figure 6.
(g)
Interpretation:
The preparation method of
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of
Explanation of Solution
In the given reaction, at first, the substrate,
The corresponding reaction sequences to obtain the desired product are shown below.
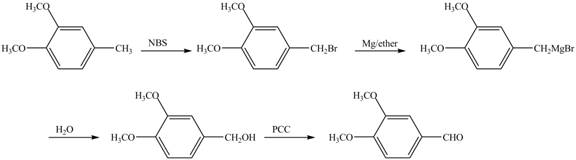
Figure 7
The preparation method of
(h)
Interpretation:
The preparation of given compound from cyclopentenol is to be given.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 17.28AP
The preparation of given compound from cyclopentenol is shown below.
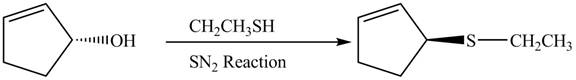
Explanation of Solution
The given reaction is a nucleophilic substitution reaction. The nucleophile attacks to replace the
This is a single step substitution nucleophile reaction as shown below.
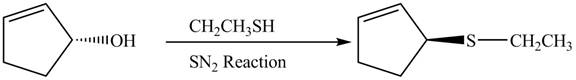
Figure 8
In this reaction, the stereochemistry of molecule changes as from one side the group is leaving and upon other side of the molecule, nucleophile attacks.
The preparation of given compound from cyclopentenol is shown in Figure 8.
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Starting with acid chloride with exactly 5 carbon atoms, and using appropriate reagents outline the synthesis of the following molecules:arrow_forwardSuggest with explanation on how you would prepare the compound, 4-chloroaniline from anilinearrow_forwardProvide the synthesis of 2-bromo-4-nitrophenyl from aniline. (structures). With explanation need.arrow_forward
- Outline the steps invloved in the synthesis of 3-chloro-4-fluroacetophenone from 4-aminoacetophenone. provide the bond line structure for the major organic product obtained in each step of the proposed synthesis.arrow_forward3) Outline an acceptable step by step mechanism for the Nucleophilic aromatic substitution of ortho-fluoronitrobenzene with methylamine. Do not forget to include the formal charges of all non-hydrogen atoms that do not have a neutral charge.arrow_forward3. Obtain acetophenone and acetaldehyde by reaction of glycols with periodic acid. Justify your answer with the reaction mechanism.arrow_forward
- Benzenediazonium carboxylate decomposes when heated to yield N2, CO2, and a reactive substance that can't be isolated. When benzenediazonium carboxylate is heated in the presence of furan, the following reaction is observe. Propose a structure for the intermediate in this reaction.arrow_forwardPyridine undergoes electrophilic aromatic substitution preferentially at the 3 position as illustrated by the synthesis of 3-nitropyridine. Under these acidic conditions, the species undergoing nitration is not pyridine, but its conjugate acid. Write resonance contributing structures for the intermediate formed by attack of NO2+ at the 2, 3, and 4 positions of the conjugate add of pyridine. From examination of these intermediates, offer an explanation for preferential nitration at the 3 position.arrow_forwardWolff-Kishner reduction of compound W gave compound A. Treatment of A with m-chloroperbenzoic acid gave B which on reduction with LiAH4 gave C. Oxidation of compound C with chromic acid gave D (C9H14O). Suggest the structures for A, B, C, and D.arrow_forward
- Suggest ways of synthesizing the following compounds, how would youmake the disconnections and what are the synthons?arrow_forwardIn two parts, outline the Electrophilic aromatic substitution for the nitration of benzene using nitric acid and sulfuric acid: A) In part I, using the curved arrow formalism, outline a mechanism for the formation of the nitronium ion, NO2+ starting from nitric acid, HNO3 and sulfuric acid, H2SO4. Do not forget to show all of the formal charges for each atom that is not zero in this mechanism. B) Starting from the nitronium ion that you generated in part “a” from above, outline the Electrophilic Aromatic Substitution of this ion with benzene.arrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)m-Nitrobenzenesulfonic acidarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





