
(a)
Interpretation:
The reaction in which
Concept introduction:
Grignard reagents are
Answer to Problem 17.45AP
The complete reaction between
![]()
Explanation of Solution
The given reaction is shown below.
![]()
Figure 1
The complete reaction is shown below.
![]()
Figure 2
The hydrolysis of Grignard reagent is shown in Figure 2 in the presence of a solvent
Therefore, the product formed is shown in Figure 2.
The complete reaction between
(b)
Interpretation:
The reaction between
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases.
Answer to Problem 17.45AP
The complete reaction between
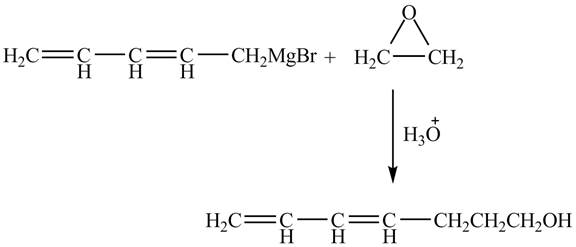
Explanation of Solution
The given reaction is shown below.
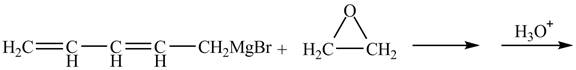
Figure 3
The complete reaction is shown below.
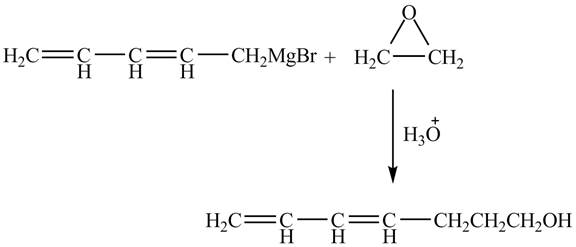
Figure 4
The Grignard reagent on treatment with epoxides in the presence of
Therefore, the product formed is shown in Figure 4.
The complete reaction between
(c)
Interpretation:
The reaction between
Concept introduction:
Nucleophiles are electron-rich species. The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic center and eliminates another group.
Answer to Problem 17.45AP
The complete reaction between
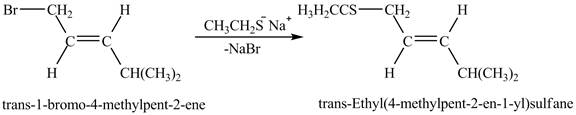
Explanation of Solution
The given reaction is shown below.
![]()
Figure 5
The complete reaction is shown below.
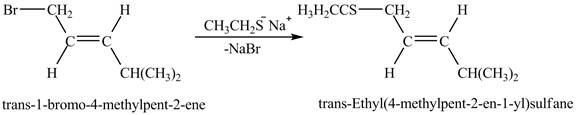
Figure 6
The above figure shows the reaction between ethyl sulfide ion and
The complete reaction between
(d)
Interpretation:
The reaction between
Concept introduction:
Nucleophiles are electron-rich species. The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic center and eliminates another group.
Answer to Problem 17.45AP
The complete reaction between
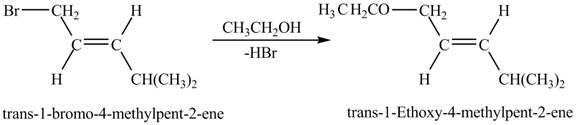
Explanation of Solution
The given reaction is shown below.

Figure 7
The complete reaction is shown below.
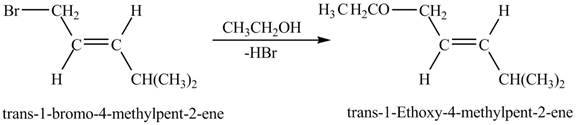
Figure 8
Figure 8 shows the reaction between
The complete reaction between
(e)
Interpretation:
The reaction of a given compound with NBS is to be completed.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl bromide, that is, bromine is substituted at the allylic position. NBS is a rich source of free radical of
Answer to Problem 17.45AP
The complete reaction of a given compound with NBS is shown below.
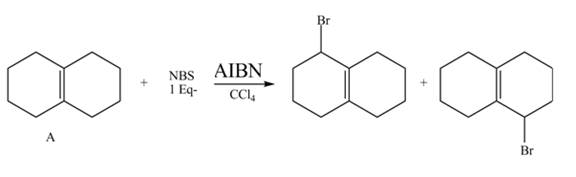
Explanation of Solution
The given reaction is shown below.
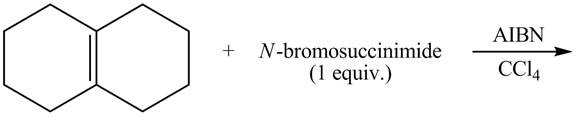
Figure 9
The complete reaction is shown below.

Figure 10
The NBS is used for allylic bromination that is it substitutes
Therefore, the products formed are shown in Figure 10.
The complete reaction of given compound with NBS is shown in Figure 10.
(f)
Interpretation:
The reaction between
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl bromide, that is, bromine is substituted at the allylic position. NBS is a rich source of free radical of
Answer to Problem 17.45AP
The complete reaction between

Explanation of Solution
The given reaction is shown below.
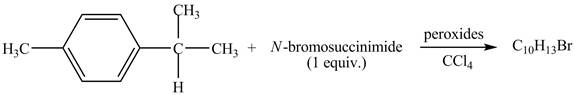
Figure 11
The complete reaction is shown below.

Figure 12
The molecular formula of the product is
Due to the addition of NBS, benzylic bromination of
Therefore, the product formed is shown in Figure 12.
The complete reaction between
(g)
Interpretation:
The complete reaction in which
Concept introduction:
The elimination reaction of alkyl halide,
Answer to Problem 17.45AP
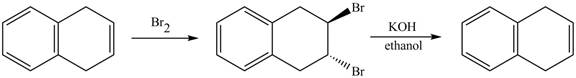
The complete reaction in which
Explanation of Solution
The given reaction is shown below.
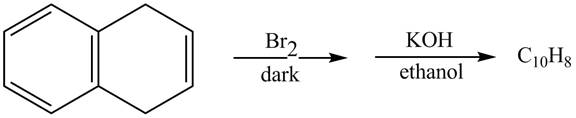
Figure 13
The complete reaction is shown below.

Figure 14
The addition of
Therefore, the product formed is shown in Figure 14.
The complete reaction in which
(h)
Interpretation:
The reaction between
Concept introduction:
When an alkene reacts with water in the acidic medium the reaction follows Markovnikov rule which states that the negative part of the reagent attacks the carbon with the least number of hydrogen atoms attached to it.
Answer to Problem 17.45AP
The complete reaction between
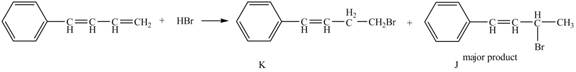
Explanation of Solution
The given reaction is shown below.
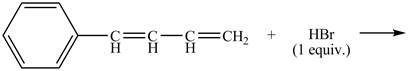
Figure 15
The complete reaction is shown below.

Figure 16
The compound,
Therefore, the products formed are shown in Figure 16.
The complete reaction between
(i)
Interpretation:
The reaction between
Concept introduction:
The substance which gets easily reduced is termed as a strong oxidizing agent. It is also defined as the substances which oxidize other substances by accepting their electrons. Examples of strong oxidizing agents are potassium permanganate, hydrogen peroxide and many more.
Answer to Problem 17.45AP
The complete reaction between
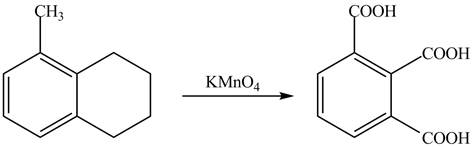
Explanation of Solution
The given reaction is shown below.
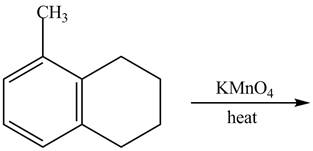
Figure 17
The complete reaction is shown below.

Figure 18
The compound,
Therefore, the product formed is shown in Figure 18.
The complete reaction between
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Give a clear handwritten answer with explanation needed...give the mechanism of given bleow reactionarrow_forwardGive a clear handwritten answer with explanation...give the mechanism of given bleow reactionarrow_forwardGive the clear handwritten answer and give the mechanism of given bleow reactionsarrow_forward
- Give a clear handwritten answer with explanation....give the products when given bleow structure reacts presence of giving each reagents ...arrow_forwardGive a clear handwritten answer with explanation..give the detailed mechanism of given bleow reaction?arrow_forwardGive the clear handwritten answer..and give synthesis mechanism of given bleow reactions...arrow_forward
- the following reaction scheme leads to the formation of compound C. give the structure of the final product C and of the intermediate products A and B and justify, using the mechanism, the formation of the product A. Give the serereochemistry of the final product obtainedarrow_forwardWhat happens when benzene and anisole react with sodium metal in the presence of ethanolic solution of ammonia. Also give mechanism for the reaction of benzene.arrow_forwardGive a clear handwritten answer with explanation. ..give below some options choose the in which given options who react with Tollen's reagent...give textual explanation also...?arrow_forward
- Give the clear handwritten answer with explanation...give the mechanism of sub parts given bleow reactions ...arrow_forwardGive a clear handwritten answer with explanation..give the mechanism of given bleow reactions...arrow_forwardGive a clear handwritten answer..with explanation..give the mechanism of given bleow reactionsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





