
(a)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering
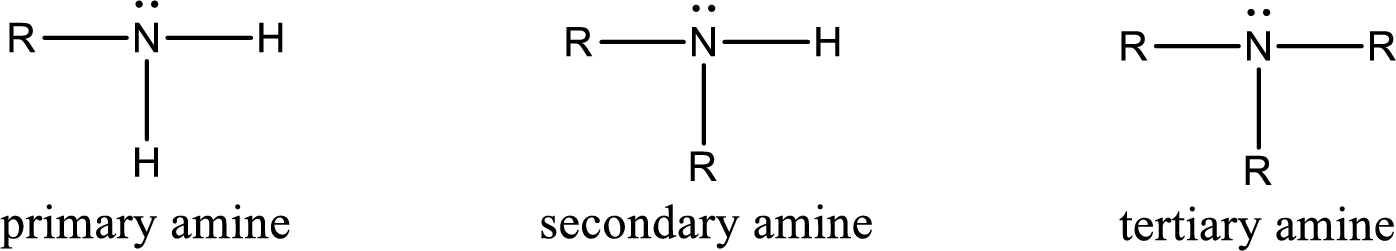
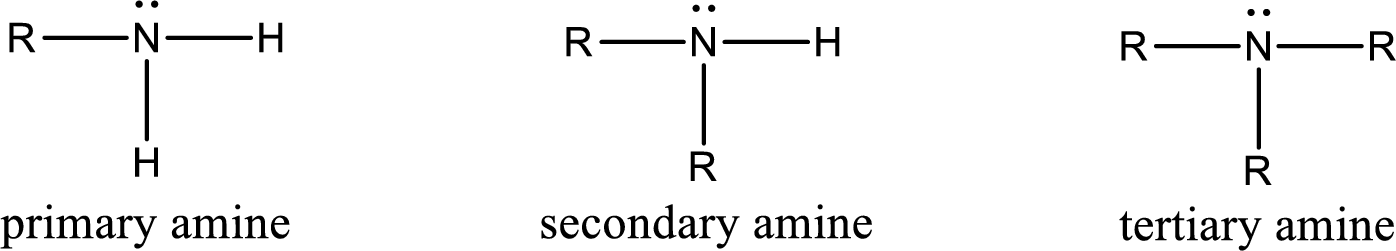
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
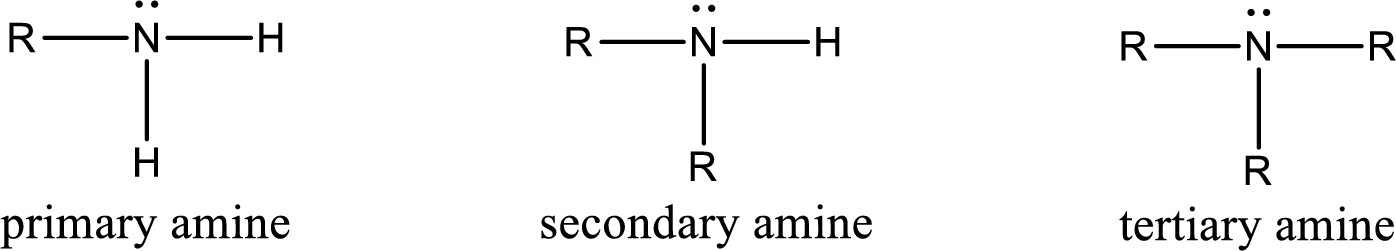
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(a)
Explanation of Solution
Given reaction is,
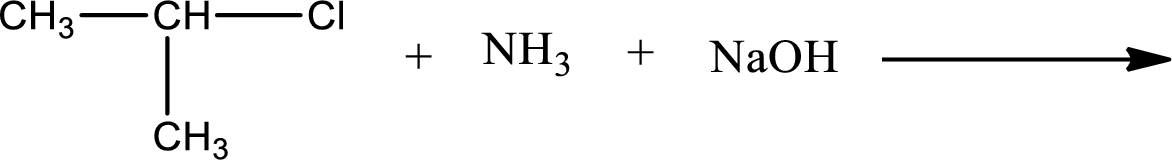
The reactants given in the above reaction are ammonia, isopropyl chloride. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between ammonia and isopropyl chloride gives isopropylamine as the product, this is an alkylation reaction. The complete reaction can be shown as,
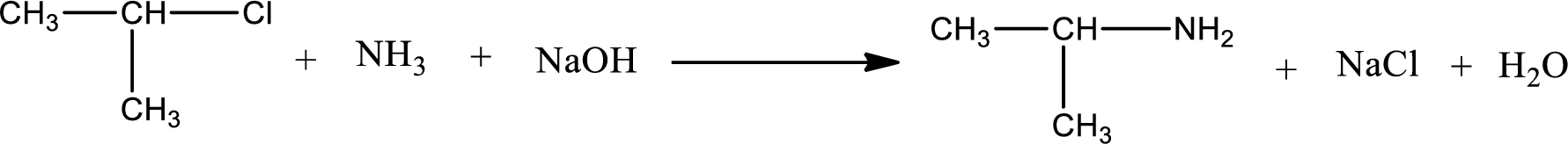
The organic product formed has a nitrogen atom that is bonded to one carbon atom and two hydrogen atoms. The inorganic product is sodium chloride and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
(b)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
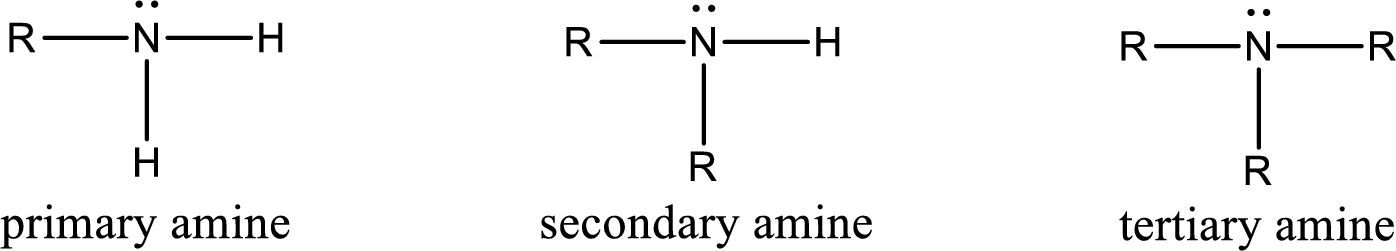
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(b)
Explanation of Solution
Given reaction is,
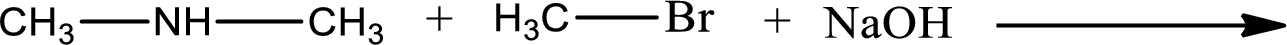
The reactants given in the above reaction are dimethylamine, methyl bromide. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between dimethylamine and methyl bromide gives trimethylamine as the product, this is an alkylation reaction. The complete reaction can be shown as,
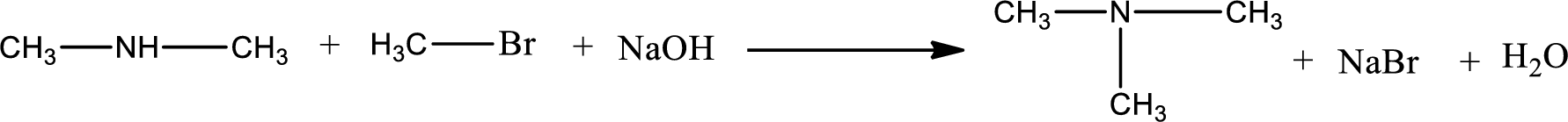
The organic product formed has a nitrogen atom that is bonded to three carbon atoms. The inorganic product is sodium bromide and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
(c)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(c)
Explanation of Solution
Given reaction is,
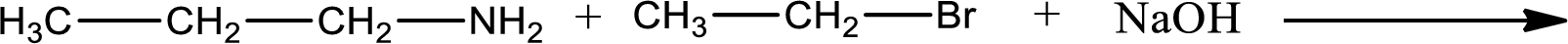
The reactants given in the above reaction are propylamine, ethyl bromide. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between propylamine and ethyl bromide gives ethylpropylamine as the product, this is an alkylation reaction. The complete reaction can be shown as,
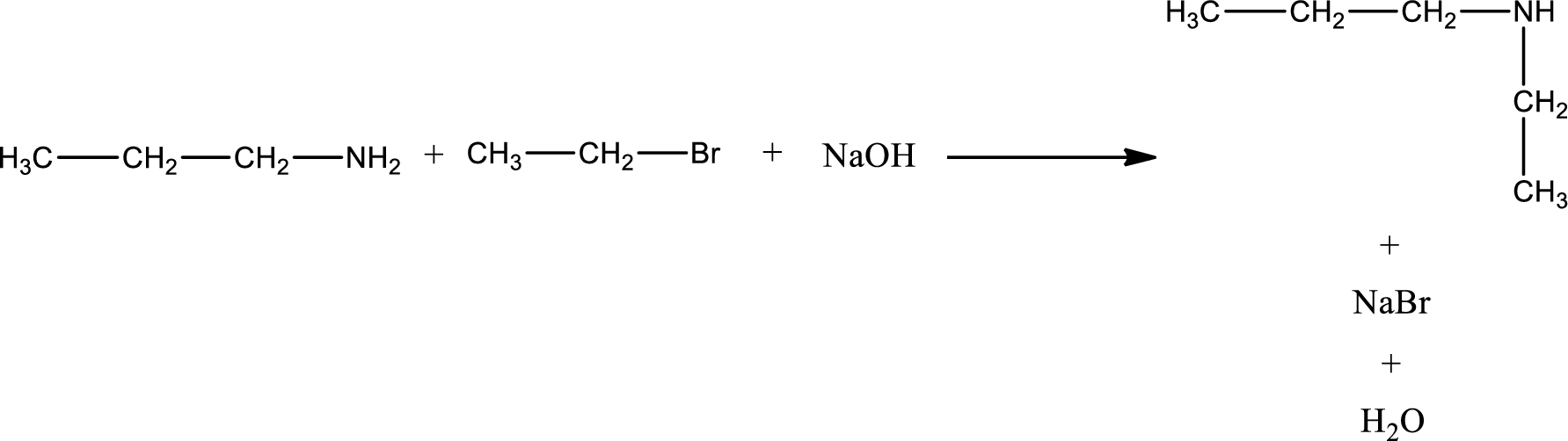
The organic product formed has a nitrogen atom that is bonded to two carbon atoms and one hydrogen atom. The inorganic product is sodium bromide and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
(d)
Interpretation:
The structure of organic product and formulas of the inorganic product formed in the given reaction has to be drawn.
Concept Introduction:
Alkylation reaction is a reaction in which the transfer of alkyl group from one molecule to another molecule takes place. While considering amines, the alkylating agent that is used is alkyl halides. Alkylation is done under basic conditions. The general equations for amines alkylation process is,
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
Quaternary ammonium salt is the one that has four carbon atoms attached to the nitrogen atom. This is formed by the reaction of tertiary amine with alkyl halide in presence of a strong base.
(d)
Explanation of Solution
Given reaction is,
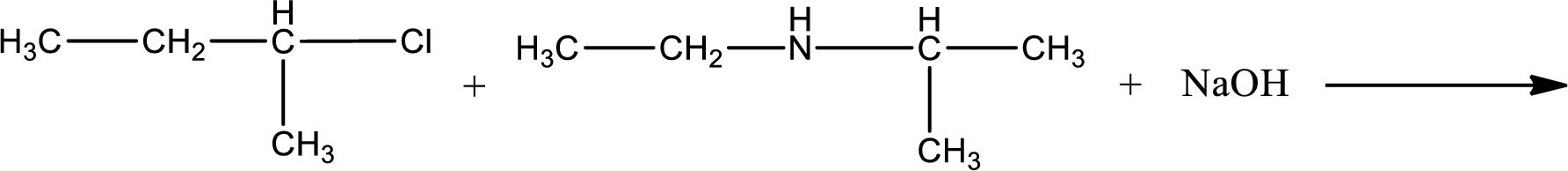
The reactants given in the above reaction are ethylisopropylamine, 2-chlorobutane. Sodium hydroxide is a reagent that is used for basic condition in this case. As the reaction between ethylisopropylamine and 2-chlorobutane gives a tertiary amine as the product, this is an alkylation reaction. The complete reaction can be given as,
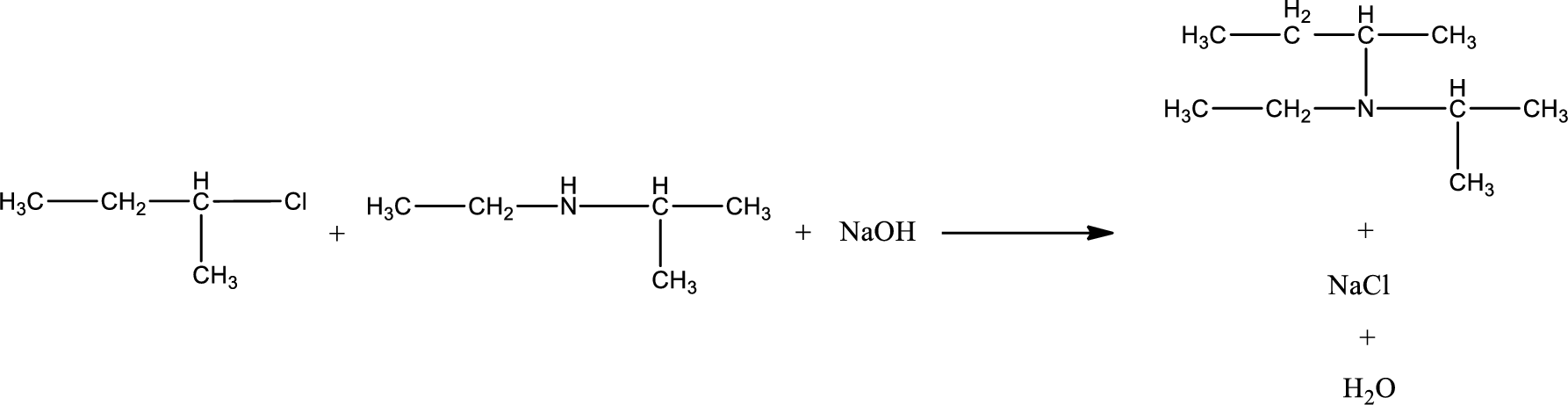
The organic product formed has a nitrogen atom that is bonded to three carbon atoms. The inorganic product is sodium chloride and water molecule. The structures are shown above.
The structure of organic product and formulas of inorganic products are drawn.
Want to see more full solutions like this?
Chapter 17 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- For the following reactions, identify the atom(s) being oxidized and reduced:arrow_forwardIn the Equation: HCO3- + HCl --------> Conjugate Acid + Conjugate Base a ) Which reactant is the Acid? b) Which reactant is the Base? c) What Conjugate Acid will form in this reaction? d) What Conjugate Base will form in this reaction?arrow_forwardWhat three important properties do catalyst have?arrow_forward
- Draw all possible carboxylic acids with the formula C5H10O2.arrow_forwardThe reaction of methoxy benzene with hydrogen iodide will yield a phenol and an alkyl halide. Which of following choices is the correct combination of the products?arrow_forwardWrite the structural formula for the product of the reaction.arrow_forward
- Consider the intermolecular forces present in a pure sample of each of the following compounds: CH₃CH₂OH and CH₃COCH₃. Identify the intermolecular forces that these compounds have in common.arrow_forwardGiven the balanced equation with an unknown compound represented by X, which compound is represented by X?arrow_forwardColumn A shows the names of some of the c functional groups. Column B shows their structure. Match each entry in column A one in column B.arrow_forward
- Which letter represents the ΔG of the reaction?arrow_forwardDraw a Fischer projection formula for the enantiomer of each of the following monosaccharides. (a to d)arrow_forwardThe linear tripeptides are formed from the following three amino acids as the starting materials in the condensation reaction. Select the correct statement: (a) There are 27 possible tripeptides and among them, 13 tripeptides are optically active. (b) There are 26 possible tripeptides and all are optically active. (c) There are 27 possible tripeptides and among them, 26 tripeptides are optically active. (d) There are 26 possible tripeptides and among them, 15 tripeptides are optically active.arrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





