
Concept explainers
17-73 Alcohols can be prepared by the acid-catalyzed hydration of
(a) Ethanol
(b) Cyclohexanol
(c) 2-Propanol
(d) 1-Phenylethanol
(a)
Interpretation:
Show the preparation of ethanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
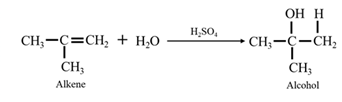
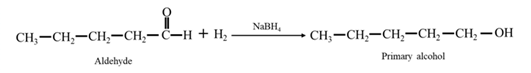
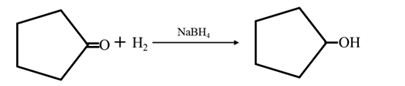
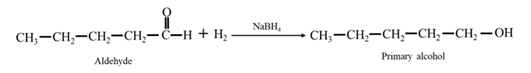
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
Answer to Problem 17.73P
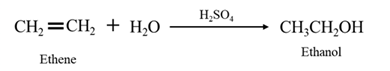
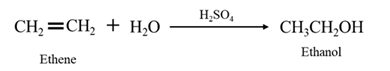
By acid-catalyzed hydration of ethane:
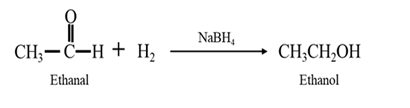
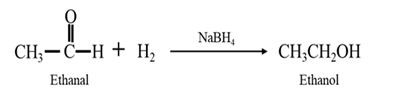
By Reduction of ethanal:
Explanation of Solution
By acid-catalyzed hydration of ethane:
When ethene is allowed to react with water in presence of an acid catalyst it gives ethanol.
By Reduction of ethanal: When ethanal is reduced in the presence of sodium borohydride it gives ethanol.
(b)
Interpretation:
Show the preparation of cyclohexanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
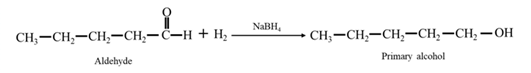
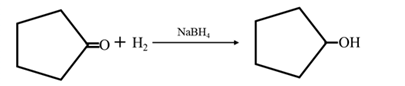
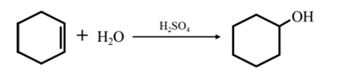
Answer to Problem 17.73P
By acid-catalyzed hydration of ethane:
By Reduction of ethanal:
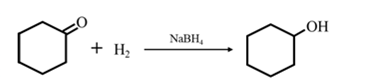
Explanation of Solution
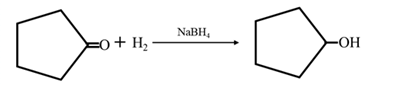
By acid-catalyzed hydration of ethane: When cyclohexene is allowed to react with water in presence of an acid catalyst it gives cyclohexanol.
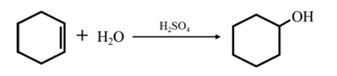
By Reduction of ethanal: When cyclohexanone is reduced in the presence of sodium borohydride it gives cyclohexanol.
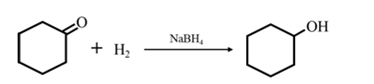
(c)
Interpretation:
Show the preparation of 2-propanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
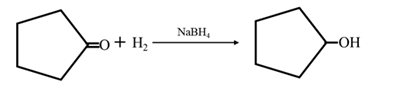
Answer to Problem 17.73P
By acid-catalyzed hydration of ethane:
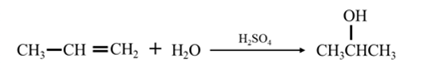
By Reduction of ethanal:
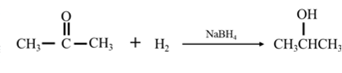
Explanation of Solution
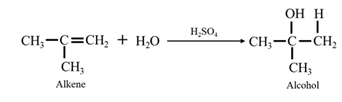
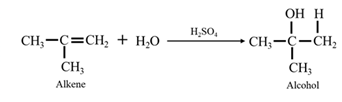
By acid-catalyzed hydration of ethane: When propene is allowed to react with water in presence of an acid catalyst it gives 2-propanol.
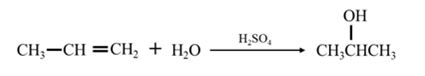
By Reduction of ethanal: When acetone is reduced in the presence of sodium borohydride it gives 2-propanol.
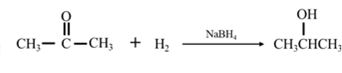
(d)
Interpretation:
Show the preparation of 1-phenylethanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
Answer to Problem 17.73P
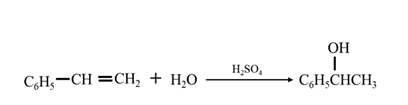
By acid-catalyzed hydration of ethane:
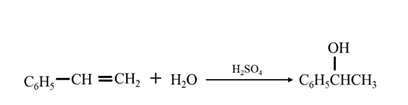
By Reduction of ethanal:
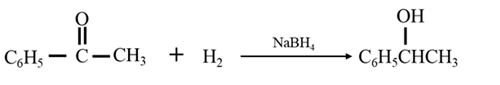
Explanation of Solution
By acid-catalyzed hydration of ethane: When 1-phenylethene is allowed to react with water in presence of an acid catalyst it gives 1-phenylethanol.
By Reduction of ethanal: When acetophenone is reduced in the presence of sodium borohydride it gives 1-phenylethanol.
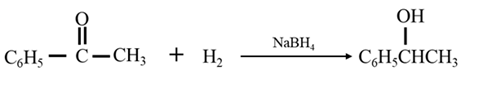
Want to see more full solutions like this?
Chapter 17 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
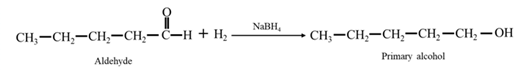
- 17-60 1-Propanol can be prepared by the reduction of an aldehyde, but it cannot be prepared by the acid catalyzed hydration of an alkene. Explain why it cannot be prepared from an alkene.arrow_forward17-70 What simple chemical test could you use to distinguish between the members of each pair of com pounds? Tell what you would do, what you would expect to observe, and how you would interpret your experimental observation. (a) Benzaldehyde and cyclohexanone (b) Acetaldehyde and acetonearrow_forward17-36 Explain why the reduction of an aldehyde always gives a primary alcohol and the reduction of a ketone always gives a secondary alcohol.arrow_forward
- 17-3 1 Draw a structural formula for the principal organic product formed when each compound is treated with K2Cr2O7/H2SO4. If there is no reaction, say so. (a) Butanal (b) Benzaldehyde (c) Cyclohexanone (d) Cyclohexanolarrow_forward17-26 Account for the fact that acetone has a higher boiling point (56°C) than ethyl methyl ether (11°C) even though their molecular weights are almost the same.arrow_forward16-28 Following is the structural formula of metformin, the hydrochloride salt of which is marketed as the antidiabetic medication Glucophage. Metformin was introduced into clinical practice in the United States in 1995 for the treatment of type 2 diabetes. More than 25 million prescriptions for this drug were written in 2000, making it the most commonly prescribed brand-name diabetes medication in the nation. NH NH H3(\ 3 N N Nh2ch3 h Metformin Complete the Lewis structure for metformin, showing all valence electrons. Which nitrogen is the most likely site of protonation? Draw the structural formula of Glucophage.arrow_forward
- 17-62 Show how to bring about these conversions. In addition to the given starting material, use any other organic or inorganic reagents as necessary. (a) 1-Pentene to 2-pentanone (b) Cyclohexene to cyclohexanonearrow_forward17-54 Following is the structure of immunosuppressant FK-506, a molecule shown to disrupt calcineurin-mediated signal transduction in T-lymphocytes. (a) There are three carbon—carbon double bonds present in this molecule. Which of the three has the potential for cis/trans isomerism? Assign a cis or trans con?guration to each carbon-carbon double bond that has this possibility. (b) How many stereocenters are present in this molecule? How many stereoisomers are possible for it? (c) Are there any aromatic components in this molecule? (d) Consider the two carbon atoms marked with asterisks. Assign an R or S con?guration of each stereocenter. (e) Because of the presence of a 21-member ring, this molecule is described as a macrocycle. This ring is fashioned by three types of bonds, several carbon-carbon bonds, one ester, one hemiacetal, and one amide. Locate the ester and the hemiacetal. (f) Draw the structural formula of the long chain compound that would result if the hemiacetal were to be cleaved to an alcohol and a carbonyl group.arrow_forward17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C) have about the same molecular weight, yet their boiling points differ by almost 50°C. Explain this fact.arrow_forward
- 2 (Chemical Connections 19A) Locate the ester group in pyrethrin I and draw a structural formula for chrysanthemic acid, the carboxylic acid from which this ester is derived.arrow_forward17-27 Pentane, 1-butanol, and butanal all have approximately the same molecular weights but different boiling points. Arrange them in order of increasing boiling point. Explain the basis for your ranking.arrow_forward17-72 The following molecule is an enediol; each carbon of the double bond carries an —OH group. Draw structural formulas for the hydroxyketone and the a-hydroxyaldehyde with which this enediol is in equilibrium.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
