
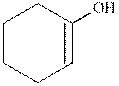
(a)
Interpretation:
The structure formula for the keto from of enol should be drawn.
Concept Introduction:
Keto and enol form both are present in the equilibrium with each other but differ in the position of hydrogen atom and double bond. These are constitutional isomers and this isomerism is known as keto- enol tautomerism.
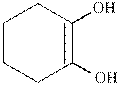
(b)
Interpretation:
The structure formula for the keto from of enol should be drawn.
Concept Introduction:
Keto and enol form both are present in the equilibrium with each other but differ in the position of hydrogen atom and double bond. These are constitutional isomers and this isomerism is known as keto- enol tautomerism.
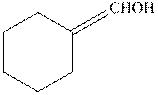
(c)
Interpretation:
The structure formula for the keto from of enol should be drawn.
Concept Introduction:
Keto and enol form both are present in the equilibrium with each other but differ in the position of hydrogen atom and double bond. These are constitutional isomers and this isomerism is known as keto- enol tautomerism.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Problem 16-4 Select the stronger base from each pair of amines.arrow_forwardProblem 17-7 Identify all hemiacetals and acetals in the following structures and tell whether each is formed from an aldehyde or a ketone. OH (a) (b) CH3OCH2CH2OCH3 (c)arrow_forwardPROBLEM 14-9Show how you would use the Williamson ether synthesis to prepare the following ethers.You may use any alcohols or phenols as your organic starting materials. 1-methoxy-4-nitrobenzenearrow_forward
- Problem 14-6 Write the common name for each ether.arrow_forwardProblem 19-2 Complete the equation for each hydrolysis reaction. Draw all products as they are ionized under these experimental conditions.arrow_forwardPROBLEM 18-28(a) Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with ethyleneglycol to give cyclohexanone ethylene acetal.(b) Propose a mechanism for the acid-catalyzed hydrolysis of cyclohexanone ethylene acetal.(c) Compare the mechanisms you drew in parts (a) and (b). How similar are these mechanisms,comparing them in reverse order?arrow_forward
- Problem 17-6 Show the reaction of benzaldehyde with one molecule of methanol to form a hemiacetal and then with a second molecule of methanol to form an acetal.arrow_forwardWhat alkenes might be used to prepare the following alcohols by hydroboration-oxidation?arrow_forwardProblem 1: It is desired to prepare polyester with the number average molecular weight, Mn- 5000 g/mol by reacting of 1 mol butane-1,4 diol (HO-CH2-CH2-CH2-CH2-OH) with 1 mol of adipic acid (HOOC-CH2-CH2-CH2-CH2-COOH). (a) Write chemical equation illustrating synthesis of the polyester. (b) Calculate the value of the monomer conversion at which the reaction should be stopped to obtain this polymer, assuming perfect stoichiometric balance. What would be polydispersity index for the polymer obtained? (c) Assuming that 0.5 mol % of the diol is lost to polymerization by a side reaction (dehydratation to a non-reactive substance), what would be the value of Mn if the reaction was carried out to the same extent as in part (b). (d) For the polymerization process value of polymerization rate constant is 1.5*102 L/(mol*s) at 100° C. How long would it take to prepare the polyester with the number average molecular weight, M, 5,000 g/mol at 100 ° C? There is no diol loss for this part of…arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

