
Concept explainers
The chapter sections to review are shown in parentheses at the end of each problem.
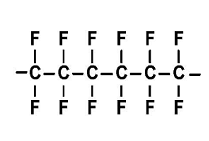
17.73 Draw a portion of the
 |
cooking pans. Teflon is used as a nonstick coating on |
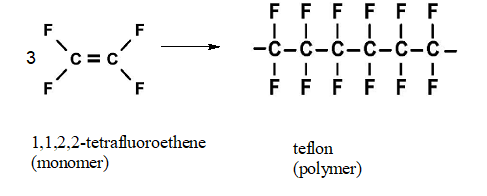
Interpretation: The expanded structural formula for a portion of teflon formed from three monomers of 1,1,2,2-tetrafluoroethene should be drawn.
Concept Introduction: The substance which is made up of molecules with a large molecular mass formed by repeating structural units which are connected by covalent bonds are said to be the polymer.
Answer to Problem 73UTC
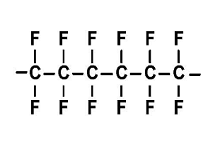
Explanation of Solution
Teflon, is a polymer in nature, made up from the 1,1,2,2-tetrafluoroethene (monomer) by the process said to be addition polymerization process
In addition polymerization process, there is no loss of any small molecule and the polymer is formed by simple linking of monomers.
Want to see more full solutions like this?
Chapter 17 Solutions
EBK BASIC CHEMISTRY
- 4 . The average human requires 120.0 grams of glucose (C6H12O6) per day. The photosynthetic reaction is: 6 CO2 + 6 H2O ---> C6H12O6 + 6 O2 How many grams of CO2 (in the photosynthesis reaction) are required for this amount of glucose?arrow_forwardA hibernating bear accumulates 25 kg of a triglyceride (846 g/mol) comprising the following fatty acids: C16, C17 and C18∆9. Calculate how many grams of β-hydroxybutyrate (104 g/mol) the bear can produce from the triglyceride during hibernation? (No other information provided).arrow_forwardA sunscreen contains 6% oxybenzone and 3% avobenzone bymass. If a bottle contains 40. g of sunscreen, how many kilogramsof oxybenzone and avobenzone are needed to manufacture1 ton of sunscreen? How many bottles of sunscreen can beproduced for 1 ton of sunscreen?arrow_forward
- Glutathione is a tripeptide whose function is to destroy harmful oxidizing agents in the body. Oxidizing agents are thought to be responsible for some of the effects of aging and to play a causative role in cancer. Glutathione removes oxidizing agents by reducing them. In the process, glutathione is oxidized, resulting in the formation of a disulfide bond between two glutathione molecules. An enzyme subsequently reduces the disulfide bond, returning glutathione to its original condition so it can react with another oxidizing agent.a. What amino acids make up glutathione? b. What is unusual about glutathione’s structure?arrow_forwardWhich is a greater source of energy as food, 5 g of fat or 9 g of carbohydrate?arrow_forwarddiabetes is a disease the body cannot maintain blood sugar (glucose) levels causing them to be high. Our bodies digest foods and breaks them down into simpler molecules, like glucose. As we know, different foods cause different glucose levels in our blood. What effect do the following have on our blood glucose levels, and why? - added sugars in foods - carbs (think starchy foods)arrow_forward
- Subject: Organic Chemistry Topic: Phases of Drug Metabolism Scenario: Y.H., a 32-year-old female with a history of chronic schizophrenia, presented for a routine follow-up visit to the behavioral health clinic. Her psychotic symptoms had been well controlled on risperidone 3 mg daily. At her previous appointment 1 month ago, Y.H. had reported being depressed, so her psychiatrist initiated a trial of paroxetine 20 mg daily as treatment. As the pharmacist was interviewing Y.H. for a medication history, he noticed that there was a resting tremor in her left hand. Y.H. acknowledged that it had first appeared within the last few weeks. She also stated that she was just starting to feel less depressed. Because both the risperidone and paroxetine appeared to demonstrate a therapeutic effect for Y.H., the pharmacist recommended that the dose of risperidone be decreased to 2 mg daily while continuing paroxetine 20 mg daily. At her next appointment the following month, Y.H. stated that her mood…arrow_forwardBelow are described three methods through which breads are leavened (risen). Which of them are chemical changes? 1. Yeast breads: Yeast added to flour and water digests carbohydrates from the flour and produces carbon dioxide (CO2) which forms bubbles in the flour water mixture. 2. Quick Breads: Flour, water, baking soda (NaHCO3) and a source of acid, are mixed and baked. The baking soda interacts with acids and heat in the batter to release carbon dioxide (CO2) gas, forming bubbles in the mixture. 3. Flat Breads: A mixture of flour and water are exposed to high heat, causing the water to evaporate quickly, forming bubbles in the mixture. A)yeast breads B)quick breads C)flat breads D)yeast breads and quick breads E)quick breads and flat breadsarrow_forwardIdentify the glycosidic bond in the following disaccharide. (b) Decide whether the compound is a non-reducing or reducing sugar. (c) Polysaccharide units are usually bonded together with a or b 1, 6 or 1, 4 linkages. What linkage is used in the disaccharide shown below?arrow_forward
- BACTROBAN ointment contains 2% w/w mupirocin. H ow many grams of a polyethylene glycol ointment base must be mixed with the contents of a 22-g tube of the BACT ROBAN ointment to prepare one having a concentration of 5 mg/g?arrow_forwardHow much of baking soda would be required to reach the LD 50 for a 159 pound human? Assuming of this compound are similarly toxic to humans.arrow_forwardSuggest a reason why some American food companies had advertised that the use of corn oil in cooking is better than the use of olive oil.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

