
(a)
Interpretation: Using the given values of heat as dependent variable, the graph should be plotted.
Concept Introduction: The energy required to change the amount of a substance from liquid to gaseous state is said to be heat of vaporization.
(a)
Answer to Problem 77A
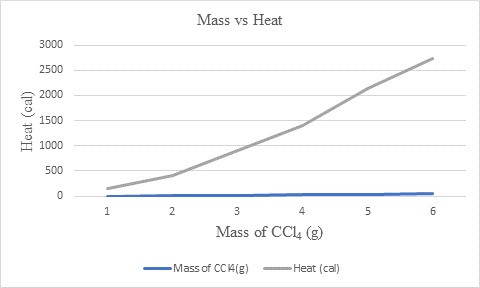
Explanation of Solution
Given information:
| Mass of CCl4(g) | Heat | |
| (J) | (cal) | |
| 2.9 | 652 | 156 |
| 7.5 | 1689 | 404 |
| 17 | 3825 | 915 |
| 26.2 | 5894 | 1410 |
| 39.8 | 8945 | 2140 |
| 51 | 11453 | 2740 |
The given values of mass and heat in cal is used to plot the graph. The x-axis represents the mass of CCl4 in grams and y-axis represents heat in cal. So,
From the graph, it can be concluded that heat (cal) is in direct relation with mass of CCl4(g) as the value of heat increases with increase in mass.
(b)
Interpretation: The slope of the line should be determined.
Concept Introduction: The formula used to find the slope of straight line is:
(b)
Answer to Problem 77A
The slope of the line is
Explanation of Solution
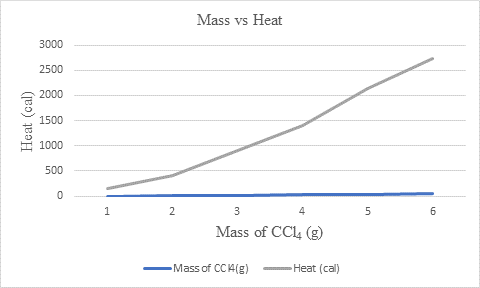
The values taken for graph plotting are:
| Mass of CCl4(g) | Heat (cal) |
| 2.9 | 156 |
| 7.5 | 404 |
| 17 | 915 |
| 26.2 | 1410 |
| 39.8 | 2140 |
| 51 | 2740 |
Now, for calculating the slope the value of
Hence, the slope of the line is
(c)
Interpretation: The comparison of heat of vaporization of CCl4(l) with the slope of line should be done.
Concept Introduction: The energy required to change the amount of a substance from liquid to gaseous state is said to be heat of vaporization.
(c)
Explanation of Solution
The heat of vaporization of CCl4(l) is
Chapter 17 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





