
(a)
Interpretation:
The structures of the
Concept introduction:
The Wilkinson’s Catalyst is a common name of coordination compound
Answer to Problem 18.15P
The structures of the transition-metal complexes involved in each of the given mechanistic step are shown below.
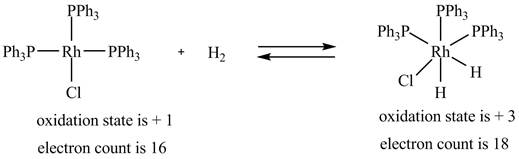
1. The oxidative addition reaction is shown below.
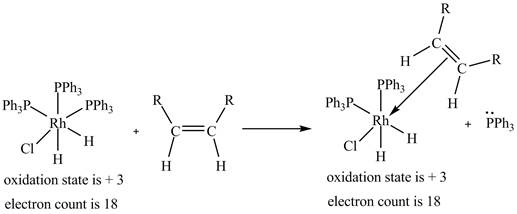
2. The ligand substitution of one
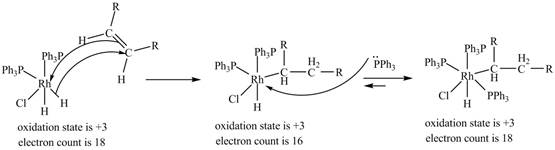
3. 1, 2-insertion of alkene into a
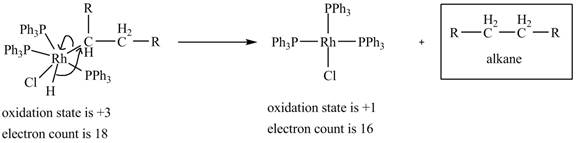
4. Reductive elimination of the
Explanation of Solution
The general formula for the calculation of electron count in a given formula is shown below.
The number of valence electrons present in rhodium is
1. In oxidative addition reaction, the central metal atom gets oxidized with the addition of two ligands and there is an increase in electron count at the central metal atom.
The oxidative addition reaction is shown below.
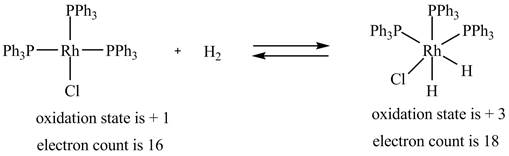
Figure 1
The electron count in
The electron count in
2. The ligand substitution reaction of one
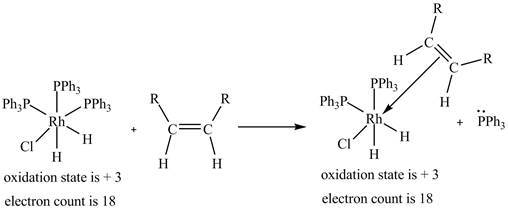
Figure 2
The electron count in
The electron count in
3. 1, 2-insertion of alkene into a
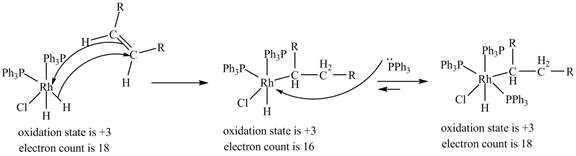
Figure 3
The electron count in
The electron count in
The electron count in
4. Reductive elimination of the alkane product to generate the catalyst is shown below.
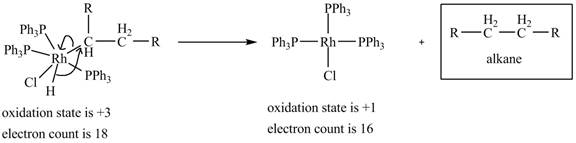
Figure 4
The electron count in
The electron count in
The catalytic hydrogenation of alkene by wilkinson’s catalyst and electron count is shown in Figure 1, 2, 3 and 4.
(b)
Interpretation:
The stereochemistry of the product if
Concept introduction:
The complex that follows
Answer to Problem 18.15P
The reaction between cis-alkene with the deuterium substituted wilkinson’s catalyst is shown below.
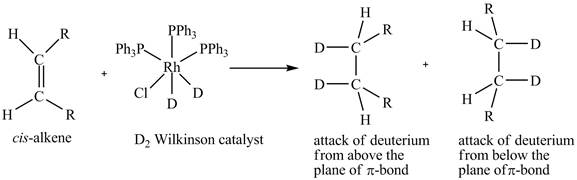
This shows syn-addition of deuterium on alkene.
Explanation of Solution
Reduction of cis-alkene with the deuterium substituted Wilkinson’s catalyst.
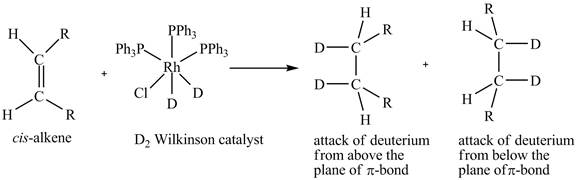
Figure 5
In the reduction of cis-alkene with the deuterium substituted Wilkinson’s catalyst. Hydrogen added on Wilkinson’s catalyst for hydrogenation of alkene is replaced by deuterium. The alkene is present in the plane of the paper, the deuterium can attack the alkene either from below the plane or above the plane.
Therefore, this shows that syn-addition of deuterium also takes place.
The reaction in Figure 5 shows that syn addition of hydrogen takes place even when it is substituted by deuterium.
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Provide a mechanism for the following reaction and rationalise the reactivity in terms of the 3 dimensional structure of the starting material and a consideration of the appropriate orbital interactions.arrow_forwardWhy cis-Ru(II)Cl2(DMSO)4 reacts with pyridine, et cetera, to give substitution of the DMSO but not the chloride ligands, but trans-Ru(II)Cl2(py)4 react with suitable Na+ and K+ salts in aqueous pyridine to afford chloride-substituted derivatives. write the reactions equations.arrow_forward(a) suggest the suitable mechanism and illustrate the stepwise of the (heptyloxy)cyclopentane mechanism. (b) illustrate, and write the mechanism the formation of iodocyclopentane and 1-iodoheptane.arrow_forward
- For each of the following mixtures of reactants, give (i) a plausible chemical equation and (ii) structurefor the organometallic product, and (iii) general reason for the course of the reaction: (a)methyllithium and W(CO)6, (b) Co2(CO)8 and AlBr3.arrow_forwardOrganotin compounds play a significant role in diverse industrial applications. They have been used as plastic stabilizers and as pesticides or fungicides. One method used to prepare simple tetraalkylstannanes is the controlled direct reaction of liquid tin(IV) chloride with highly reactive trialkylaluminum compounds, such as liquid triethylaluminum (Al(C2H5)3). 3SnCl4 + 4AI(C2H5)3 →3Sn(C2H5)4 + 4AlCl3 In one experiment, 0.160 L of SnCl4 (d = 2.226 g/mL) was treated with 0.346 L of triethylaluminum (Al(C2H5)3): d = 0.835 g/mL). What is the theoretical yield in this experiment (mass of tetraethylstannane, Sn(C2H5)4)? If 0.257 L of tetraethylstannane (d= 1.187 g/mL) were actually isolated in this experiment, what was the percent yield?arrow_forwardConsider the reaction of two compounds ‘A’ and ‘B’ which could make two possible diastereomers ‘AB’ and ‘BA’ (much like this week’s Diels Alder reaction). Hand-write your calculations and responses to the following questions and upload your work as a .jpg or .pdf file. Which of the two products (A or B) will form in greater abundance under thermodynamic control? Which will form in greater abundance under kinetic control? Explain your responses using a sketch of the reaction coordinate diagram for the reactions described above.arrow_forward
- Provide a reasonable synthetic strategy for the synthesis of a racemic mixture of (1R,2R) and (1S,2S)-2-bromo-1-methylcyclopentanol from the compound shown below... Provide the bond line structure for the major organic products obtained in each step of the proposed strategyarrow_forwardCompound A(C10H12O)gives off oxygen on treatment with sodium metal and also decolorizes Br2 in CCl4 to give organic compound B. Compound A on treatment with I2 in NaOH gives iodoform and salt C which after acidification gives a white solid D(C7H6O2). Using knowledge of organic chemistry identify structures A,B,C and Darrow_forwardWhich of the following reaction coordinate diagrams represents SN1 and E1 reactions? A B C Darrow_forward
- give UV-vis spectrum showing (n to pi*) transition in addition to the main one and HNMR spectrum of the product from the following reaction and explain one possible reactions by this reaction scheme.arrow_forwardHydrocarbon S, C8H8 reacts with hydrogen in the presence of platinum catalyst to yield T, C8H10. Compound U is formed when T is heated with alkaline potassium permanganate solution, followed by hydrolysis. The reaction of S with hydrogen bromide V, whereas ozonolysis of S produces W and methanal. Draw the structures of S to W. Give the name of the reaction for the conversion of S to T. Write all chemical equations involved.arrow_forwardProvide an explanation for this order of reactivity.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





