Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.7P
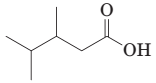
18-7 Write the IUPAC name for each carboxylic acid.

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Give an IUPAC and common name for each of the following naturally occurring carboxylic acids: (a) CH3CH(OH)CO2H (lactic acid); (b) HOCH2CH2C(OH)(CH3)CH2CO2H (mevalonic acid).
Summarize the IUPAC nomenclature rules for namingcarboxylic acids.
Chapter 18 Solutions
Introduction to General, Organic and Biochemistry
Ch. 18.2 - Prob. 18.1PCh. 18.5 - Prob. 18.2PCh. 18.5 - Prob. 18.3PCh. 18 - 18-4 Answer true or false. (a) The functional...Ch. 18 - Prob. 18.5PCh. 18 - 18-6 Name and draw structural formulas for the...Ch. 18 - 18-7 Write the IUPAC name for each carboxylic...Ch. 18 - 18-8 Write the IUPAC name for each carboxylic...Ch. 18 - Prob. 18.9PCh. 18 - Prob. 18.10P
Ch. 18 - Prob. 18.11PCh. 18 - Prob. 18.12PCh. 18 - Prob. 18.13PCh. 18 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 18 - 18-15 Draw a structural formula for the dimer...Ch. 18 - 18-16 Propanedioic (malonic) acid forms an...Ch. 18 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 18 - 18-18 Propanoic acid and methyl acetate are...Ch. 18 - 18-19 The following compounds have approximately...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - 18-23 Characterize the structural features...Ch. 18 - Prob. 18.24PCh. 18 - Prob. 18.25PCh. 18 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 18 - Prob. 18.27PCh. 18 - 18-28 Arrange these compounds in order of...Ch. 18 - 18-29 Complete the equations for these acid—base...Ch. 18 - 18-30 Complete the equations for these acid-base...Ch. 18 - 18-31 Formic acid is one of the components...Ch. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Prob. 18.36PCh. 18 - Prob. 18.37PCh. 18 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - 18-41 Complete these examples of Fischer...Ch. 18 - Prob. 18.42PCh. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 18 - 18-47 Methylparaben and propylparaben are used as...Ch. 18 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 18 - Prob. 18.49PCh. 18 - Prob. 18.50PCh. 18 - Prob. 18.51PCh. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - Prob. 18.55P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 18-19 The following compounds have approximately the same molecular weight: hexanoic acid, heptanal, and 1-heptanol. Arrange them in order of increasing boiling point.arrow_forward18-31 Formic acid is one of the components responsible for the sting of biting ants and is injected under the skin by bee and wasp stings. The pain can be relieved by rubbing the area of the sting with a paste of baking soda (NaHCO3) and water, which neutralizes the acid. Write an equation for this reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY