
(a)
Interpretation:
The structure for the eight constitutional isomers of molecular formula C4H11N should be drawn.
Concept Introduction:
There are three types of
Answer to Problem 42P
The structures for the eight constitutional isomers of molecular formula C4H11N are represented as follows:

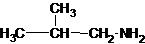
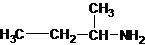
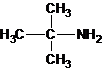
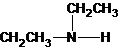
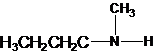
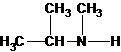
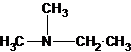
Explanation of Solution
Four structures of primary amines can be drawn with the formula C4H11N.

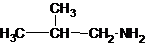
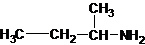
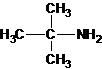
Three structures of secondary amines can also be drawn.
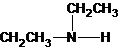
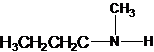
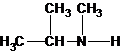
A tertiary structure can also be drawn as follows:
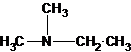
(b)
Interpretation:
The systematic name for each amine should be given.
Concept Introduction:
In nomenclature of primary amine, the longest carbon chain bonded to nitrogen is determined and the −e ending of the parent
Answer to Problem 42P
The name of amines are as follows:

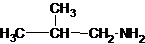
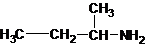
1-butanamine 2-methylpropan-1-amine butan-2-amine
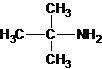
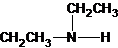
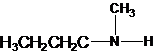
2-methylpropan-2-amine N-ethylethanamine N-methylpropan-1-amine
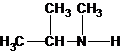
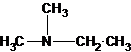
N-methylpropan-2-amine N,N-dimethylethanamine
Explanation of Solution

The longest carbon chain has four carbons. So the alkane name is butane. N is attached to C-1. Therefore, the systematic name of the amine is butanamine.
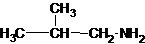
The longest carbon chain has three carbons. There is a methyl group at C-2. So the parent name is 2-methylpropanamine. The N atom is bonded to C-1. Therefore, the name become 2-methylpropan-1-amine.
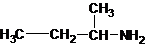
The longest carbon chain bonded to amine group has four carbons. The parent name is butanamine. The N atom is bonded to C-2. Therefore, the systematic name of the amine is butan-2-amine.
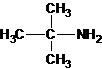
The longest carbon chain bonded to amine group has three carbons. There is a methyl group at C-2. The parent name is 2-methylpropanamine. The N atom is bonded to C-2. Therefore, the systematic name of the amine is 2-methylpropan-2-amine.
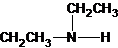
The secondary amine has the longest carbon chain with 2 carbons. So, the parent name is ethanamine. The N atom has bonded to C-1 and has 1 ethyl group as a substituent. Therefore, the systematic name become N-ethylethanamine.
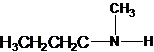
The secondary amine has the longest carbon chain with 3 carbons. So, the parent name is propanamine. The N atom has bonded to C-1 and has 1 methyl group as a substituent. Therefore, the systematic name become N-methylpropan-1-amine.
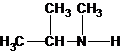
The secondary amine has the longest carbon chain with 3 carbons. So, the parent name is propanamine. The N atom has bonded to C-2 and has 1 methyl group as a substituent. Therefore, the systematic name become N-methylpropan-2-amine.
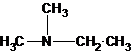
The tertiary amine has the longest carbon chain with 2 carbons. So the parent name is ethanamine. N atom has bonded to C-1 and has two methyl groups and 1 ethyl group as substituents. So, the systematic name of the amine is N,N-dimethylethanamine.
(c)
Interpretation:
The chirality center present in one of the amines should be identified.
Concept Introduction:
An atom that has four different groups bonded to it is referred to as chirality center. A chiral molecule has a non-superimposable mirror image.
Answer to Problem 42P
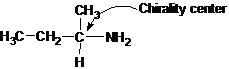
Explanation of Solution
Butan-2-amine has long carbon chain with 4 carbons and amine group is bonded to C-2. This C-2 carbon has four different groups bonded to it as 1 ethyl group, 1 methyl group, 1 amine group and a hydrogen. So, C-2 carbon is a chirality center.
Want to see more full solutions like this?
Chapter 18 Solutions
GENERAL ORGANIC & BIOCHEMISTRY >ACCESS<
- A) Name the following amine. H3C−CH2−CH2−NH−CH2−CH2−CH3 Spell out the full name of the compound. B ) Name the following amine. CH3−CH2−NH−CH2−CH2−CH3 Spell out the full name of the compound.arrow_forwardWhat is the molecular Shape of the Pharmacutical drug Ativan?arrow_forwardN-Methylpyrrolidine has a boiling point of 81 °C, and piperidine has a boiling point of 106 °C. Tetrahydropyran has a boiling point of 88 °C, and cyclopentanone has a boiling point of 141 °C. These two isomershave a boiling point difference of 53 °C. Explain why the two oxygen-containing isomers have a much larger boilingpoint difference than the two amine isomersarrow_forward
- a.Primary amides tend to exist as dimers in the solid and liquid state. b.Dimethylacetamide, CH3CON(CH3)2 has a higher boiling point than acetamide. c.Nitrile is often classified as an acid derivative because it is hydrolyzed to a -COOH. d.Esters have lower boiling points than ketones of comparable molecular masses. Whice are correct?arrow_forwardAssume for the purposes of this problem that to be an alcohol (-ol) or an amine (-amine), the hydroxyl or amino group must be bonded to a tetrahedral (sp3 hybridized) carbon atom. Write the structural formula of a compound with an unbranched chain of four carbon atoms that is an: Q. Alkynoic acidarrow_forwardIs it possible for primary and secondary amines to act as very weak acids? Explain.arrow_forward
- Draw the structural formula for a compound with the given molecular formula. Q.)A chiral quaternary ammonium salt, C6H16NClarrow_forwardAssume for the purposes of this problem that to be an alcohol (-ol) or an amine (-amine), the hydroxyl or amino group must be bonded to a tetrahedral (sp3 hybridized) carbon atom. Write the structural formula of a compound with an unbranched chain of four carbon atoms that is an: Q. Alkenoic acidarrow_forwardDraw structural formulas for Q.) The four primary (1°) amines with the molecular formula C4H11N.arrow_forward
- Considering Hinsberg's Method to determine, 1°, 2°, 3° amines. Draw the structure of the products of the following reactions. Aniline (1°) + BSC, excess NaOH(DRAW STRUCTURE), then, + HCl (DRAW STRUCTURE) Diethylamine (2°)+ BSC, excess NaOH (DRAW STRUCTURE), then, + HCl (DRAW STRUCTURE) Triethylamine (3°)+ BSC, excess NaOH(DRAW STRUCTURE), then, + HCl (DRAW STRUCTURE)arrow_forwardDraw structures of four different amides with molecular formula C3H7NO.arrow_forwardDraw the structure of a compound of molecular formula C4H11NO that ts each description: (a) a compound that contains a 1° amine and a 3° alcohol; (b) a compound that contains a 3° amine and a 1° alcohol.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



