
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
8th Edition
ISBN: 9781305864504
Author: Brent L. Iverson, Sheila Iverson
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.8, Problem DQ
The following sequence of steps is used to create the
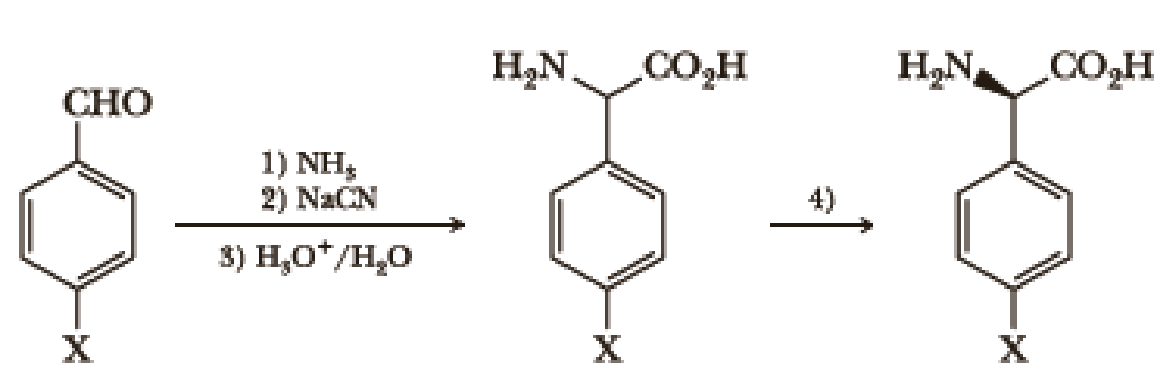
- 1. Step 1 would best be performed at a pH below the pKa of ammonium.
- 2. Step 1 would create an imine that undergoes nucleophilic attack by cyanide in Step 2.
- 3. The hydrolysis of the nitrile created in Step 2 would be better performed in base rather than the acid shown.
- 4. Step 4 is a resolution of a racemic mixture that results from Steps 1–3 and does not require a chiral reagent.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles.
Identify the product that is formed when the conjugate base of diethyl malonate reacts with the following
electrophile followed by acid workup when relevant:
Modify the provided structures of diethyl malonate and the given electrophile to draw the product formed.
Note: the eraser tool can be used to erase bonds, and atoms can be moved by selecting them with the selector
tool and then dragging the selected atom(s) to a new position. If you make a mistake, you can use Ctrl-Z or the
Undo tool.
бль
EtO
OEt
Edit Drawing
The hydrolysis of the ester shown here is catalyzed by morpholine. Explain how morpholine catalyzes the reaction. (Hint: The pKa of the conjugateacid of morpholine is 9.3, so morpholine is too weak a base to function as a base catalyst.)
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is
formed when the conjugate base of diethyl malonate reacts with the following electrophile followed by acid workup when relevant:
Modify the provided structures of diethyl malonate and the given electrophile to draw the product formed. Note: the eraser tool can
be used to erase bonds, and atoms can be moved by selecting them with the selector tool and then dragging the selected atom(s) to a
new position. If you make a mistake, you can use Ctrl-Z or the Undo tool.
OH
-CH₂
CH₂
Edit Drawing
Chapter 18 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
Ch. 18.1 - Prob. 18.1PCh. 18.2 - Prob. 18.2PCh. 18.4 - Prob. 18.3PCh. 18.4 - Prob. 18.4PCh. 18.4 - Synthesis of nitriles by nucleophilic displacement...Ch. 18.5 - Complete the following transesterification...Ch. 18.6 - Complete and balance equations for the following...Ch. 18.8 - Prob. AQCh. 18.8 - Several compounds have been found to inhibit...Ch. 18.8 - Prob. CQ
Ch. 18.8 - The following sequence of steps is used to create...Ch. 18.9 - Prob. 18.8PCh. 18.9 - Prob. 18.9PCh. 18.10 - Prob. 18.10PCh. 18.10 - Show how to convert (R)-2-phenylpropanoic acid to...Ch. 18 - Prob. 18.12PCh. 18 - Write the IUPAC name for each compound. (a)...Ch. 18 - Prob. 18.14PCh. 18 - Prob. 18.15PCh. 18 - Propose a structural formula for compound A,...Ch. 18 - Propose a structural formula for compound B,...Ch. 18 - Propose a structural formula for each compound...Ch. 18 - Draw a structural formula for the principal...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - Prob. 18.23PCh. 18 - Prob. 18.24PCh. 18 - Show the product expected when the following...Ch. 18 - The reagent diisobutylaluminum hydride (DIBALH)...Ch. 18 - Prob. 18.27PCh. 18 - Prob. 18.28PCh. 18 - Prob. 18.29PCh. 18 - Nicotinic acid, more commonly named niacin, is one...Ch. 18 - Prob. 18.31PCh. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Isoniazid, a drug used to treat tuberculosis, is...Ch. 18 - Prob. 18.37PCh. 18 - A step in a synthesis of PGE1 (prostaglandin E1,...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - Show how to synthesize 5-nonanone from...Ch. 18 - Prob. 18.42PCh. 18 - The following sequence of steps converts...Ch. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - Prob. 18.46PCh. 18 - Prob. 18.47PCh. 18 - Following is a retrosynthetic analysis for the...Ch. 18 - Prob. 18.50PCh. 18 - Given this retrosynthetic analysis, propose a...Ch. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - In Problem 7.28, we saw this step in Johnsons...Ch. 18 - Prob. 18.56PCh. 18 - Prob. 18.57PCh. 18 - Prob. 18.58PCh. 18 - Prob. 18.59PCh. 18 - Prob. 18.60PCh. 18 - Prob. 18.61PCh. 18 - Prob. 18.62PCh. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Using your reaction roadmap as a guide, show how...Ch. 18 - Minoxidil is a molecule that causes hair growth in...Ch. 18 - Prob. 18.69PCh. 18 - Prob. 18.70PCh. 18 - Prob. 18.71P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
The structural formula of 1, 2-dimethylbenzene needs to be drawn. Concept introduction: The ring structures of ...
Chemistry: Matter and Change
What is the pH range for acidic solutions? For basic solutions?
Introduction to Chemistry
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living By Chemistry: First Edition Textbook
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry
During the early part of the 20th century, sulfanilamide (an antibacterial drug) was only administered by injec...
Elementary Principles of Chemical Processes, Binder Ready Version
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with the following electrophile followed by acid workup when relevant: CN Modify the provided structures of diethyl malonate and the given electrophile to draw the product formed. Note: the eraser tool can be used to erase bonds, and atoms can be moved by selecting them with the selector tool and then dragging the selected atom(s) to a new position. If you make a mistake, you can use Ctrl-Z or the Undo tool. EtO H3C CN Edit Drawing OEtarrow_forwardGiven the information pictured, The final step of the reaction sequence uses PhLi. Provide a reason and possible mechanism by which this final elimination and hydrolysis reaction would occur.arrow_forwardOut of acid chlorides (least stable), anhydrides, esters, and amides (most stable). True or false Acid chlorides would be the most reactive to hydrolysis and Amides would be the least reactivearrow_forward
- Imine hydrolysis is rapid under strongly acidic conditions (H20, 1 equivalent HCI), but imine formation is slow under the same conditions. Provide a reasonable explanation for the observed reactivity. NH +H20, 1 eq. HCI NH3 FAST NH -H20, 1 eq. HCI NH3 SLOW Enter your answer herearrow_forward3. You are given a mixture of aspirin, phenol, and naphthalene that you need to separate. i) Draw the structures of each, and identify if they are acidic, basic, or neutral compounds. For each compound draw their reaction with the appropriate acidic or basic conditions that will change their solubility and allow them to be separated. ii) What modifications would you have to make to the experimental protocol in order to separate these three compounds? Provide specifics.arrow_forwardComplete the following proposed acid–base reactions, and predict whether the reactants or products are favored.arrow_forward
- 2. Your friend is also in the lab and they are running a series of organic reactions with the HCI from your reaction. a) Your friend is trying to figure out how to form N-ethyl-4-methylhex-2-amine from the compound below. They know they can use the HCI in their reaction, but asked for your help to understand the synthesis pathway. Determine the reactions that would take place with the HCI, the compound above and any other organic compounds or reagents needed to form N-ethyl-4-methylhex-2-amine. b) Consider the compound used in the reaction (image provided above in a)): i) What is the chemical formula? ii) What is the oxidation state of each element in the formula? c) Select one compound in reaction in part a. Draw the compound and label the hybridization states of each element on your image. Identify all inter and intramolecular forces present in a solution of your selected compound. Selected Compound: Hybridization States (insert image)arrow_forwardIdentify whether the hydroxide ion is functioning as a base or as a nucleophile.arrow_forwardTwo methods convert an alkyl halide to a carboxylic acid having one more carbon atom. Depending on the structure of the alkyl halide, one or both of these methods may be employed. For each alkyl halide, write out a stepwise sequence that converts it to a carboxylic acid with one more carbon atom. If both methods work, draw both routes. If one method cannot be used, state why it can't.arrow_forward
- The product in this reaction is basic enough to be protonated by a dilute HCl solution. Draw the protonated species, clearly showing where protonation occurs. Draw all possible resonance structures of the conjugate acid of the product, and use these to explain why the product is so much more basic than a typical ester, like ethyl acetate.arrow_forward1. Give the order of basicity of alkaloids based on the R-groups attached to the amino functional group. Based on your knowledge in organic chemistry, explain the reason why one is more or less basic as compared to the other.arrow_forwardWrite the mechanism for the following reactions: 1. the acid-catalyzed hydrolysis of an imine to a carbonyl compound and a primary amine 2. the acid-catalyzed hydrolysis of an enamine to a carbonyl compound and a secondary amine a. How do the two mechanisms differ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License