
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.9, Problem 16P
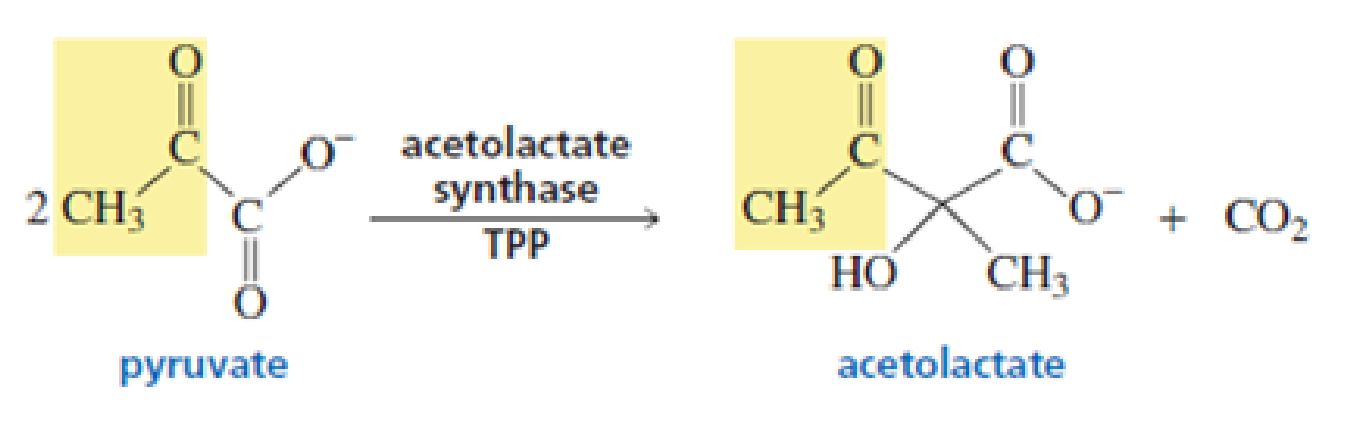
Acetolactate synthase is another TPP-requiring enzyme. It, too, catalyzes the decarboxylation of pyruvate, but it transfers the resulting acyl group to another molecule of pyruvate, forming acetolactate. This is the first step in the biosynthesis of the amino acids valine and leucine. Propose a mechanism for this reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Acetolactate synthase is another TPP-requiring enzyme. It transfers the acyl group of pyruvate to another molecule of pyruvate, forming acetolactate. This is the first step in the biosynthesis of the amino acids valine and leucine. Propose a mechanism for this reaction.
The phosphoryl group on phosphoglucomutase is slowly lost by hydrolysis. Propose a mechanism that utilizes a known catalytic intermediate for restoring this essential phosphoryl group. How might this phosphoryl donor be formed?
Imidazoleglycerol‑phosphate dehydratase is an enzyme in the histine biosynthesis pathway. It catalyzes the E1 dehydration of D‑erthyro‑imidazole‑glycerol phosphate to imidazole acetol‑phosphate. This is a rare example of a biological E1 reaction, as most biological elimination reactions occur through E1cB instead.
In this reaction, D‑erthyro‑imidazole‑glycerol phosphate is first protonated to form a good leaving group. Then, the leaving group is ejected to form the resonance‑stabilized carbocation shown. Draw curved arrows forming the most stable resonance structure to explain why this reaction goes through an E1 mechanism.
Draw curved arrows to form the most stable resonance structure.
Chapter 18 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 18.1 - Prob. 1PCh. 18.2 - If H218O were used to hydrolyze lysozyme, which...Ch. 18.3 - Which of the following amino acid side chains can...Ch. 18.3 - Arginine and lysine side chains fit into trypsins...Ch. 18.4 - Which of the following amino acid side chains can...Ch. 18.4 - Prob. 6PCh. 18.5 - Prob. 7PCh. 18.5 - Draw the mechanism for the hydroxide-ion-catalyzed...Ch. 18.5 - What advantage does the enzyme gain by forming an...Ch. 18.7 - Prob. 10P
Ch. 18.7 - Prob. 11PCh. 18.8 - How many conjugated double bonds are there in a....Ch. 18.8 - Instead of adding to the 4a-position and...Ch. 18.8 - In succinate dehydrogenase, FAD is covalently...Ch. 18.8 - Prob. 15PCh. 18.9 - Acetolactate synthase is another TPP-requiring...Ch. 18.9 - Acetolactate synthase can also transfer the acyl...Ch. 18.9 - Prob. 18PCh. 18.9 - Prob. 19PCh. 18.10 - Prob. 21PCh. 18.11 - Prob. 23PCh. 18.11 - Which compound is more easily decarboxylated?Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.12 - What groups are interchanged in the following...Ch. 18.13 - Why is the coenzyme called tetrahydrofolate?Ch. 18.13 - What amino acid is formed by the following...Ch. 18.13 - How do the structures of tetrahydrofolate and...Ch. 18.13 - What is the source of the methyl group in...Ch. 18 - Prob. 32PCh. 18 - Prob. 33PCh. 18 - From what vitamins are the following coenzymes...Ch. 18 - Prob. 35PCh. 18 - For each of the following reaction, name both the...Ch. 18 - Explain why serine proteases do not catalyze...Ch. 18 - Prob. 38PCh. 18 - For each of the following enzyme catalyzed...Ch. 18 - Trisephosphate isomerase (TIM) catalyzes the...Ch. 18 - Prob. 41PCh. 18 - What acyl groups have we seen transferred by...Ch. 18 - When UMP is dissolved in T2O, exchange of T for H...Ch. 18 - Prob. 44PCh. 18 - When transaminated, the three branched-chain amino...Ch. 18 - Aldolase shows no activity if it is incubated with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of xylulose 5-phosphate with ribose 5-phosphate in the presence of a transketolase to give glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate. (a) The first part of the reaction is nucleophilic addition of thiamin diphosphate (TPP) ylide to xylulose 5-phosphate, followed by a retro-aldol cleavage to give glyceraldehyde 3-phosphate and a TPPcontaining enamine. Show the structure of the enamine and the mechanism by which it is formed. (b) The second part of the reaction is addition of the enamine to ribose 5-phosphate followed by loss of TPP ylide to give sedoheptulose 7-phosphate. Show the mechanism.arrow_forwardTriosephosphate isomerase (TIM) catalyzes the conversion of dihydroxyacetone phosphate to glyceraldehyde-3-phosphate. The enzyme’s catalytic groups are Glu 165 and His 95. In the first step of the reaction, these catalytic groups function as a general-base and a general-acid catalyst, respectively. Propose a mechanism for the reaction.arrow_forwardPyruvate carboxylase is thought to activate CO2 by ATP, through formation of carboxyphosphate as an intermediate. Propose a mechanism for the formation of this intermediate.arrow_forward
- lysozyme catalyzes the hydrolysis of a carbohydrate linkage in part of the bacterial wall. The active site of the lysozyme contains aspartate and glutamate side chains. 1. lysozyme catalyzes this reaction with a catalytic Zn 2+ ion. propose a mechanism.arrow_forwardThe same E1–E2–E3 multienzyme structure found in the pyruvate dehydrogenase and the a-ketoglutarate dehydrogenase complexes is also used in the branched-chain a-ketoacid dehydrogenase complex, which participates in the catabolism of branched-chain amino acids. Draw the reaction product when the following substrate is acted on by the branched-chain a-keto acid dehydrogenase complex.arrow_forwardThe following compound has been found to be an inhibitor of penicillinase. The enzyme can be reactivated by hydroxylamine (NH2OH). Propose a mechanism to account for the inhibition and for the reactivation.arrow_forward
- Carbonic anhydrase is an enzyme that catalyzes the conversion of carbon dioxide to bicarbonate ion. It is a metalloenzyme, with Zn2+ coordinated at the active site by three histidine side chains. Propose a mechanism for the reaction.arrow_forwardThe given mechanism of transamination reaction is shown below, To complete the shuffling of amino groups and carbonyls, the PMP must be converted back to PLP. How would you predict that this occurs? 1. A separate reaction oxidizes the amine group of PMP to an aldehyde. 2. A separate reaction hydrolyzes the amine of PMP to an aldehyde. 3. A different ketoacid reacts with the amine of PMP, and the entire sequence runs backward. 4. Either 2 or 3 could occur.arrow_forwardOne of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of sedoheptulose 7-phosphate with glyceraldehydes 3-phosphate in the presence of a transaldolase to yield erythrose 4-phosphate and fructose 6-phosphate. (a) The first part of the reaction is the formation of a protonated Schiff base of sedoheptulose 7-phosphate with a lysine residue in the enzyme followed by a retro-aldol cleavage to give an enamine plus erythrose 4-phosphate. Show the structure of the enamine and the mechanism by which it is formed. (b) The second part of the reaction is a nucleophilic addition of the enamine to glyceraldehyde 3-phosphate followed by hydrolysis of the Schiff base to give fructose 6-phosphate. Show the mechanism.arrow_forward
- The vitamin Niacin is used to form nicotinamide adenosine dinucleotide, which readily shuttles between its oxidized (NAD+) and reduced (NADH) forms. The latter serves as a cellular equivalent to NaBH4. The essential portions of the structures are shown below. Outline a mechanism for the cellular conversion of pyruvate to lactate. (Note: like NaBH4, NADH cannot reduce carboxylic acid carbonyls).arrow_forwardAcetolactate synthase transfers the acyl group of pyruvate to alpha-ketobutyrate. This is the first step in the biosynthesis of the amino acid isoleucine. Propose a mechanism for this reaction.arrow_forwardWhat type of reaction would a PLP-containing enzyme catalyze if the bond most perpendicular to PLP was a C-COO- bond? A、racemization B、transamination C、aldol cleavage D、decarboxylationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY